
How is phenol manufactured by the Cumene process?
Answer
582.6k+ views
Hint: Cumene process is a widely used method for the production of phenol. It involves synthesizing two relatively cheap compounds, viz. Benzene and propylene, into two extremely useful products like phenol and acetone.
Complete step by step answer:
Before we move forward with the solution of the question, let us understand some basic concepts first:
-Phenols are cyclic organic compounds that are formed when one or more sites on a benzene ring, substitute their hydrogen atoms in exchange for the corresponding number of -OH or alcoholic functional groups. Now, phenols are formed as a product in various reactions, but it is not possible to completely extract all of the phenol formed from these reactions.
Hence, certain methods are developed just to maximise the extraction of phenol from a given chemical reaction. One such process is known as the Cumene process.
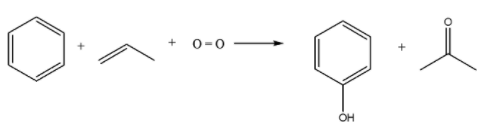
-Cumene process for manufacturing phenol has many names including cumene – phenol process and Hock process. This method is used for producing both phenol and acetone. The reactants used in this reaction are benzene and propylene. Another important reactant required is the oxygen, which is obtained from the air. Also, to be included is a small amount of radical initiators. This method is used all over the world for the production of phenol. The chemical reaction involved in this process is as follows:
Note:
The most important chemical made from phenol is bisphenol A, which is used to make the polycarbonates. Phenol is also catalytically reduced to cyclohexanol, which is used in the manufacture of polyamides 6 and 6,6.
Complete step by step answer:
Before we move forward with the solution of the question, let us understand some basic concepts first:
-Phenols are cyclic organic compounds that are formed when one or more sites on a benzene ring, substitute their hydrogen atoms in exchange for the corresponding number of -OH or alcoholic functional groups. Now, phenols are formed as a product in various reactions, but it is not possible to completely extract all of the phenol formed from these reactions.
Hence, certain methods are developed just to maximise the extraction of phenol from a given chemical reaction. One such process is known as the Cumene process.
-Cumene process for manufacturing phenol has many names including cumene – phenol process and Hock process. This method is used for producing both phenol and acetone. The reactants used in this reaction are benzene and propylene. Another important reactant required is the oxygen, which is obtained from the air. Also, to be included is a small amount of radical initiators. This method is used all over the world for the production of phenol. The chemical reaction involved in this process is as follows:

Note:
The most important chemical made from phenol is bisphenol A, which is used to make the polycarbonates. Phenol is also catalytically reduced to cyclohexanol, which is used in the manufacture of polyamides 6 and 6,6.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




