
Phenol, when it first reacts with concentrated sulphuric acid and then with concentrated nitric acid, gives
(A) 2,4,6-trinitrobenzene
(B) o-nitrophenol
(C) p-nitrophenol
(D) nitrobenzene
Answer
582.3k+ views
Hint: The -OH group attached to the benzene ring in phenol has the effect of making the ring much more reactive than it would otherwise be. It also reacts with dilute nitric acid, whereas benzene itself needs a nitrating mixture of concentrated nitric acid and concentrated sulfuric acid.
Complete answer:
We have been provided with phenol,
Phenol is an aromatic organic compound with the molecular formula ${{C}_ {6}} {{H}_ {5}} OH$,
When phenol reacts with concentrated sulphuric acid,
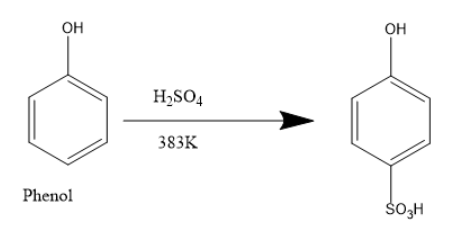
Phenol reacts with sulfuric acid giving substituted phenols. At room temperature an equilibrium is established between a mixture of phenol sulphuric acid and the product benzene ortho hydroxy sulfuric acid.
And then the product benzene ortho hydroxy sulfuric acid reacts with concentrated nitric acid,
Phenol on reaction with conc. ${{H}_ {2}} S{{O}_ {4}} $ gives a mixture of o- and p- products and then at room temperature o- product is more stable, which on treatment with conc. $HN{{O}_ {3}} $ will yield o-nitrophenol. Since temperature is not mentioned, o-nitrophenol is the only stable product.
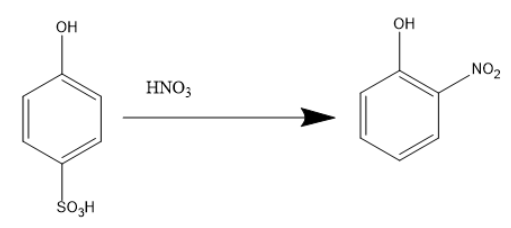
When benzene ortho hydroxy sulfuric acid reacts with concentrated nitric acid ortho-nitrophenol is formed.
Therefore, we can conclude that option (B) is correct.
Note:
Ortho-nitrophenol is stabilized by intramolecular Hydrogen-bonding between the H-atom of OH group and an oxygen of the nitro-group. Para-nitrophenol lacks such extra stability and hence, is relatively less stable than o-nitrophenol. Hence, the major product is o-nitrophenol.
Complete answer:
We have been provided with phenol,
Phenol is an aromatic organic compound with the molecular formula ${{C}_ {6}} {{H}_ {5}} OH$,
When phenol reacts with concentrated sulphuric acid,

Phenol reacts with sulfuric acid giving substituted phenols. At room temperature an equilibrium is established between a mixture of phenol sulphuric acid and the product benzene ortho hydroxy sulfuric acid.
And then the product benzene ortho hydroxy sulfuric acid reacts with concentrated nitric acid,
Phenol on reaction with conc. ${{H}_ {2}} S{{O}_ {4}} $ gives a mixture of o- and p- products and then at room temperature o- product is more stable, which on treatment with conc. $HN{{O}_ {3}} $ will yield o-nitrophenol. Since temperature is not mentioned, o-nitrophenol is the only stable product.

When benzene ortho hydroxy sulfuric acid reacts with concentrated nitric acid ortho-nitrophenol is formed.
Therefore, we can conclude that option (B) is correct.
Note:
Ortho-nitrophenol is stabilized by intramolecular Hydrogen-bonding between the H-atom of OH group and an oxygen of the nitro-group. Para-nitrophenol lacks such extra stability and hence, is relatively less stable than o-nitrophenol. Hence, the major product is o-nitrophenol.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




