
p-Hydroxy benzophenone upon reaction with bromine in carbon tetrachloride gives:
(A)
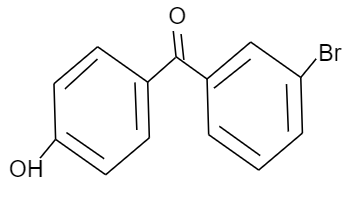
(B)
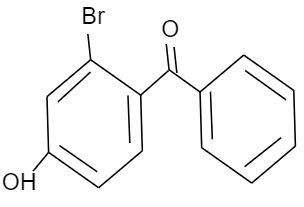
(C)
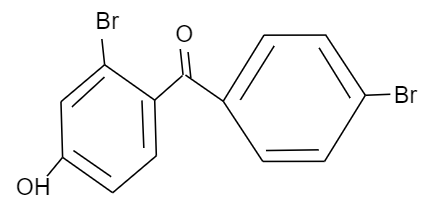
(D)
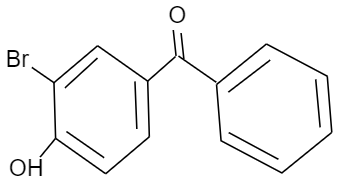




Answer
577.2k+ views
Hint: You should know that the oxygen atom of the hydroxyl group present in the compound given has lone pairs of electrons. So, consider the hydroxyl group will undergo a positive mesomeric effect, where the ortho and para positions get activated for the electrophilic substitution reaction.
Complete step by step solution:
Given that, p-hydroxy benzophenone which has chemical formula ${{C}_{13}}{{H}_{10}}{{O}_{2}}$ is being reacted with bromine in presence of carbon tetrachloride (having chemical formula $CC{{l}_{4}}$). We have to find out the major product formed by this reaction. We should know that, the hydroxyl group present in the p-hydroxy benzophenone will show mesomeric effect and it will specifically show positive mesomeric effect because of the presence of lone pairs of electrons on the oxygen atom of hydroxyl group in the structure. Due to this positive mesomeric effect, the ortho and para sites in the structure of the compound given will get activated towards the electrophilic substitution reaction. As we know, bromine is an electrophile component. So, it will get directed to attack on the ortho and para position of the structure of the compound given. But as the ortho position is already occupied by the hydroxyl group in the structure, it will get directed towards the para position of the structure forming a major product known by the name $3-\text{bromo}-4-\text{hydroxyphenyl phenyl methanone}$.
The reaction is shown as follows:
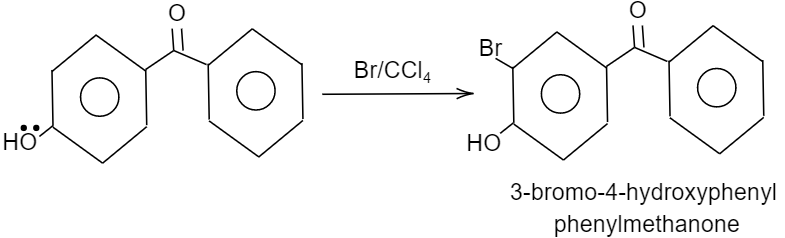
Hence, the correct option is D.
Note: You can get confused with option A where the bromine attacks the para position of another benzene ring, but it should be noted that the electrophile will attack that group having the lone pairs of electrons. Remember when the pi electrons are moved from the conjugate system to a group, negative mesomeric effect takes place while when the pi electrons moved from a particular group to a conjugate system, positive mesomeric effect takes place and for positive mesomeric effect, the group should have either a lone pair of electrons or a negative charge upon it.
Complete step by step solution:
Given that, p-hydroxy benzophenone which has chemical formula ${{C}_{13}}{{H}_{10}}{{O}_{2}}$ is being reacted with bromine in presence of carbon tetrachloride (having chemical formula $CC{{l}_{4}}$). We have to find out the major product formed by this reaction. We should know that, the hydroxyl group present in the p-hydroxy benzophenone will show mesomeric effect and it will specifically show positive mesomeric effect because of the presence of lone pairs of electrons on the oxygen atom of hydroxyl group in the structure. Due to this positive mesomeric effect, the ortho and para sites in the structure of the compound given will get activated towards the electrophilic substitution reaction. As we know, bromine is an electrophile component. So, it will get directed to attack on the ortho and para position of the structure of the compound given. But as the ortho position is already occupied by the hydroxyl group in the structure, it will get directed towards the para position of the structure forming a major product known by the name $3-\text{bromo}-4-\text{hydroxyphenyl phenyl methanone}$.
The reaction is shown as follows:

Hence, the correct option is D.
Note: You can get confused with option A where the bromine attacks the para position of another benzene ring, but it should be noted that the electrophile will attack that group having the lone pairs of electrons. Remember when the pi electrons are moved from the conjugate system to a group, negative mesomeric effect takes place while when the pi electrons moved from a particular group to a conjugate system, positive mesomeric effect takes place and for positive mesomeric effect, the group should have either a lone pair of electrons or a negative charge upon it.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




