
Please explain how we can differentiate beta and alpha-D-fructopyranose from beta and alpha-L-fructofuranose? What is the difference in their ring structures?
Answer
561.3k+ views
Hint: Beta and alpha structures differ by the position of –OH in the anomeric carbon.
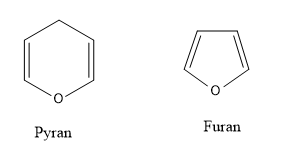
- Pyranose is analogue to pyran and furanose is analogue to furan.
Complete step by step answer:
So let’s analyse the question given, here in the question, it is asked that we have to comment on the beta and alpha structures of D-fructopyranose and beta and alpha structures of L-fructofuranose.
- First we should know the difference between the alpha and the beta structures. Both the structures differ by the position of the hydroxyl group (-OH) present in the anomeric carbon.
If the –OH group is present pointing downwards the ring then the structure is called as alpha structure and if the structure is –OH is pointing upwards then the structure is a beta structure.
Now we have to know the difference between fructopyranose and fructofuranose.
Fructopyranose is a structure which is analogous to the cyclic structure called as pyran, which is a 6 carbon membered ring.
Fructofuranose is a structure which is analogous to the cyclic structure called furan which is a 5 membered ring.
The structures of pyran and furan are as follows:
By gathering all these information let's draw the structure of alpha and beta structures of D-fructopyranose and alpha and beta structure of D-fructofuranose.
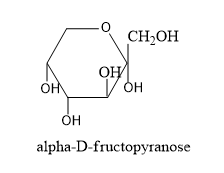
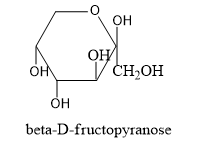
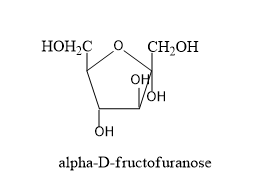
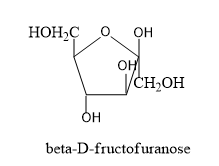
- Now we have to know the difference in structures of L isomers and D-isomers.
L-isomers are the structures which are mirror images of the D-isomers.
To draw the structures of the L-isomers we have to change the configuration of each carbon. We have to interchange the configuration of the groups. Those groups that are pointing upwards should be written downwards and vice versa.
Hence let’s trace the structure of L-fructofuranose by following the above said conditions.
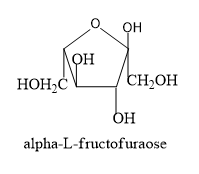
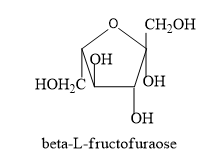
Note: In the D- isomers the –OH group is placed on the right side of the asymmetric carbon which is placed furthest from the carbonyl group whereas in the L-isomer the –OH group is placed on the left side.
- Pyranose is analogue to pyran and furanose is analogue to furan.
Complete step by step answer:
So let’s analyse the question given, here in the question, it is asked that we have to comment on the beta and alpha structures of D-fructopyranose and beta and alpha structures of L-fructofuranose.
- First we should know the difference between the alpha and the beta structures. Both the structures differ by the position of the hydroxyl group (-OH) present in the anomeric carbon.
If the –OH group is present pointing downwards the ring then the structure is called as alpha structure and if the structure is –OH is pointing upwards then the structure is a beta structure.
Now we have to know the difference between fructopyranose and fructofuranose.
Fructopyranose is a structure which is analogous to the cyclic structure called as pyran, which is a 6 carbon membered ring.
Fructofuranose is a structure which is analogous to the cyclic structure called furan which is a 5 membered ring.
The structures of pyran and furan are as follows:

By gathering all these information let's draw the structure of alpha and beta structures of D-fructopyranose and alpha and beta structure of D-fructofuranose.




- Now we have to know the difference in structures of L isomers and D-isomers.
L-isomers are the structures which are mirror images of the D-isomers.
To draw the structures of the L-isomers we have to change the configuration of each carbon. We have to interchange the configuration of the groups. Those groups that are pointing upwards should be written downwards and vice versa.
Hence let’s trace the structure of L-fructofuranose by following the above said conditions.


Note: In the D- isomers the –OH group is placed on the right side of the asymmetric carbon which is placed furthest from the carbonyl group whereas in the L-isomer the –OH group is placed on the left side.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




