
Predict the products formed when cyclohexane carbaldehyde reacts with the following reagents.
Semicarbazide and weak acid
Answer
566.4k+ views
Hint: The structure of cyclohexane carbaldehyde is as follows.
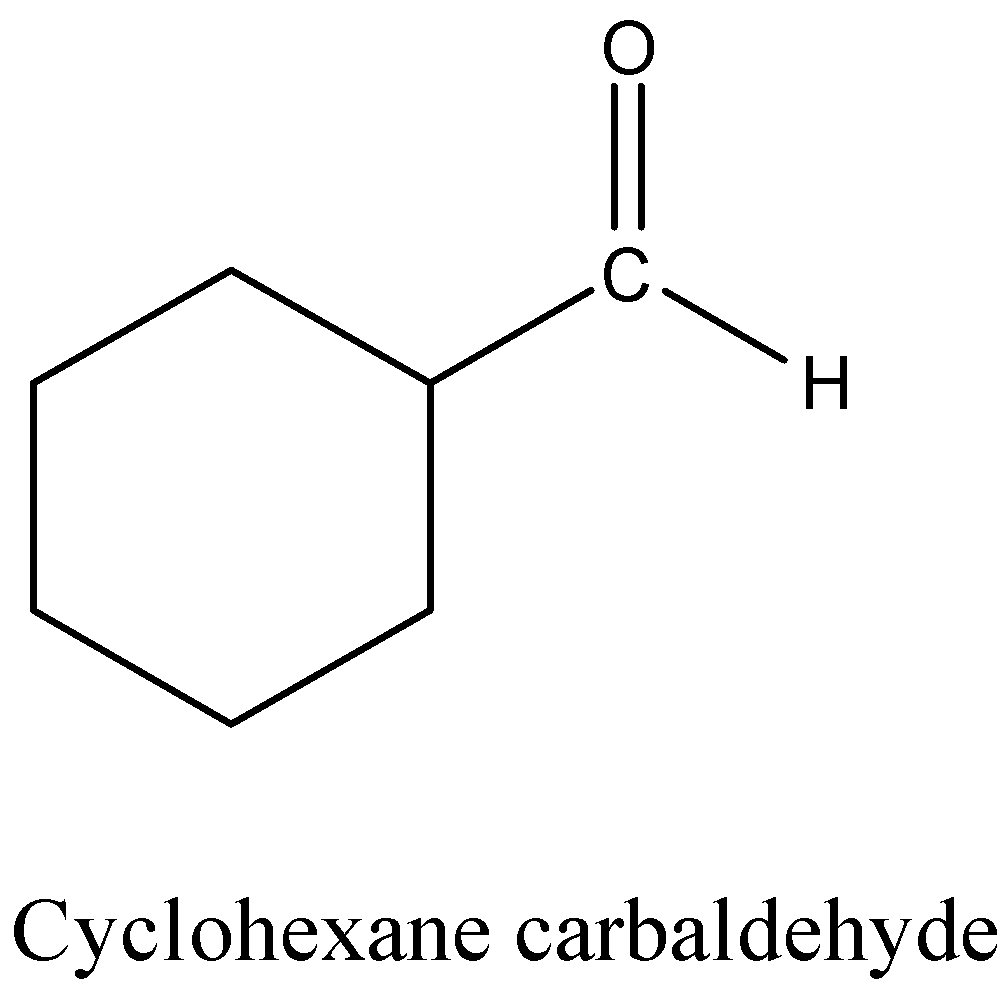
- The structure of semicarbazide is as follows.
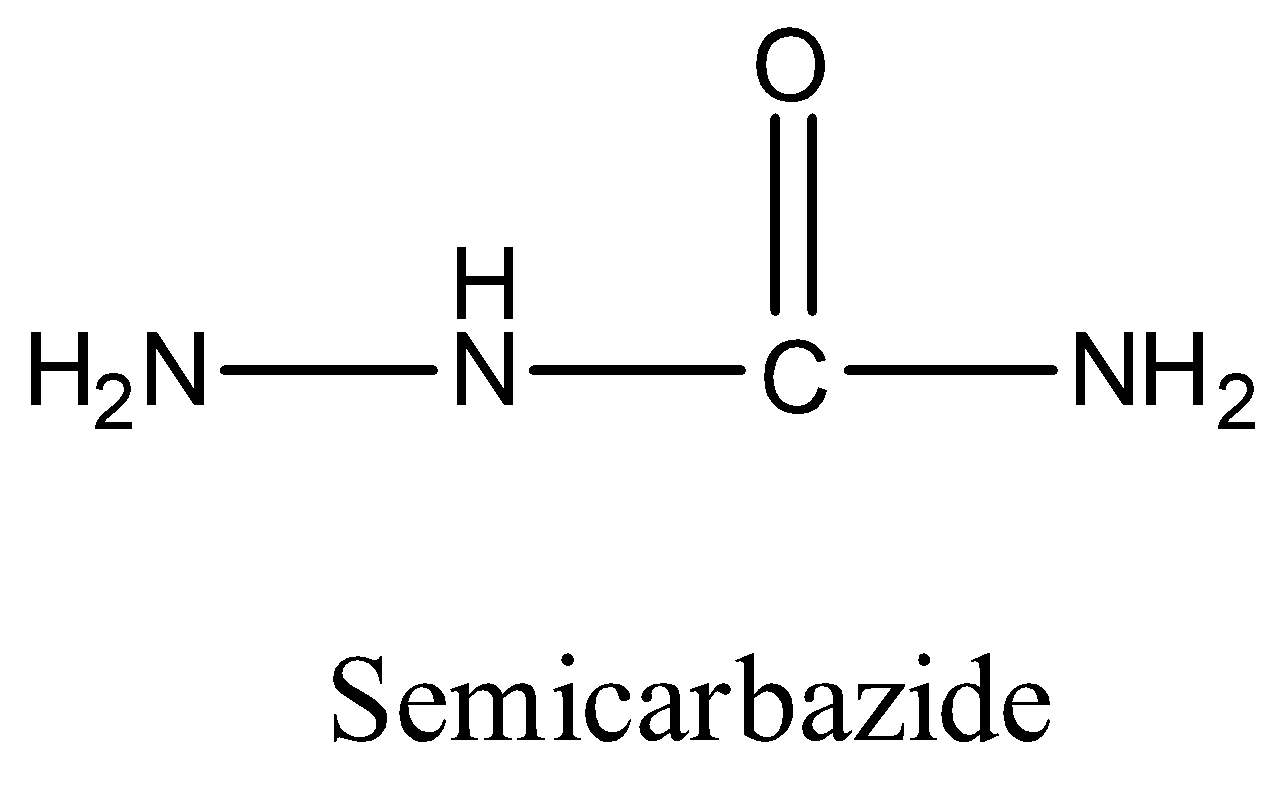
In the structure of the semicarbazide there are three amines, out of those three amines two amines are in resonance with carbonyl carbon and amine (terminal) is free from resonance.
Complete answer:
- In the question it is given that to find the products formed when cyclohexane carbaldehyde reacts with semicarbazide and a weak acid.
- We have to find the products when cyclohexane carbaldehyde reacts with semicarbazide and a weak acid.
- The chemical reaction of cyclohexane carbaldehyde with semicarbazide in presence of a weak acid is as follows.
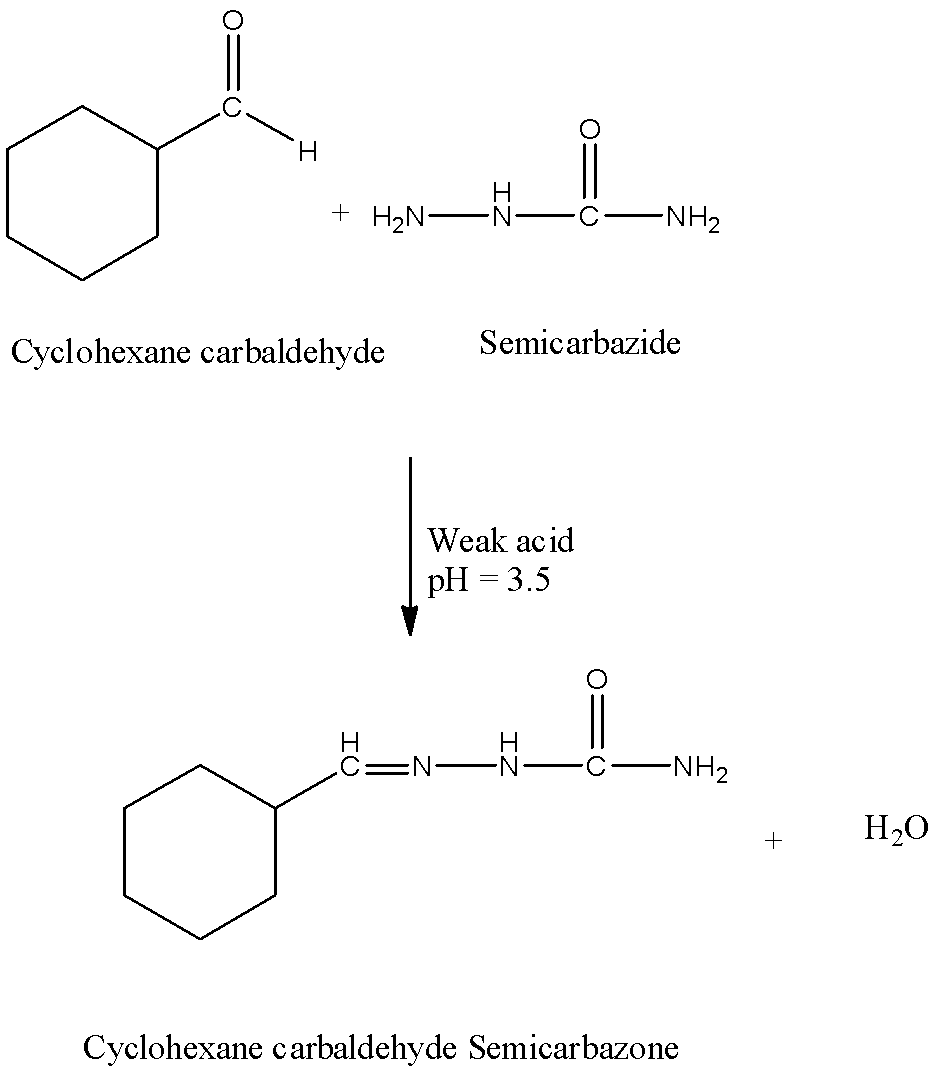
- In the above reaction cyclohexane carbaldehyde reacts with semicarbazide in presence of a weak acid (pH = 3.5) and forms a product called Cyclohexane carbaldehyde Semicarbazone.
- Therefore the product formed is Cyclohexane carbaldehyde Semicarbazone and water.
Additional information:
- The reaction between cyclohexane carbaldehyde and semicarbazide is only possible in acidic conditions.
- There is a need for a weak acid (pH = 3.5) to make the reaction occur, in basic conditions the reaction does not happen.
Note: Generally in semicarbazide there are three amines. Out of those three amines one amine is not in resonance with the carbonyl group and it is going to act as a nucleophile. The amine which is not in resonance with the carbonyl group in semicarbazide is going to initiate the reaction.

- The structure of semicarbazide is as follows.

In the structure of the semicarbazide there are three amines, out of those three amines two amines are in resonance with carbonyl carbon and amine (terminal) is free from resonance.
Complete answer:
- In the question it is given that to find the products formed when cyclohexane carbaldehyde reacts with semicarbazide and a weak acid.
- We have to find the products when cyclohexane carbaldehyde reacts with semicarbazide and a weak acid.
- The chemical reaction of cyclohexane carbaldehyde with semicarbazide in presence of a weak acid is as follows.

- In the above reaction cyclohexane carbaldehyde reacts with semicarbazide in presence of a weak acid (pH = 3.5) and forms a product called Cyclohexane carbaldehyde Semicarbazone.
- Therefore the product formed is Cyclohexane carbaldehyde Semicarbazone and water.
Additional information:
- The reaction between cyclohexane carbaldehyde and semicarbazide is only possible in acidic conditions.
- There is a need for a weak acid (pH = 3.5) to make the reaction occur, in basic conditions the reaction does not happen.
Note: Generally in semicarbazide there are three amines. Out of those three amines one amine is not in resonance with the carbonyl group and it is going to act as a nucleophile. The amine which is not in resonance with the carbonyl group in semicarbazide is going to initiate the reaction.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




