
How will you prepare 2-methyl-propan-2-ol from methyl magnesium bromide?
Give a balance equation.
Answer
584.7k+ views
Hint: We must remember that the Grignard reagent is a chemical compound having general formula R-Mg-X where X represents halogen atoms and R represents alkyl or aryl groups. Grignard reagent is used for the synthesis of hydrocarbon, alcohols, carboxylic acids etc.
Complete step by step answer:
We must know in the laboratory, Grignard reagent plays a major role in the synthesis of aldehydes from alcohol. In Grignard reagent, Mg metal is bonded with alkyl or aryl group by highly polar covalent bond and Mg bonded with halogen by ionic bond. Examples of Grignard reagents are methyl magnesium chloride $\left( {C{H_3}MgCl} \right)$, methyl magnesium bromide $\left( {C{H_3}MgBr} \right)$and phenyl magnesium bromide $\left( {{C_6}{H_5}MgBr} \right)$ which are collectively called as organo magnesium compound.
For the preparation of 2-methyl-propan-2-ol from methyl magnesium bromide, first we react the methyl magnesium bromide with propanone (having molecular formula${\text{C}}{{\text{H}}_3} - {\text{CO}} - {\text{C}}{{\text{H}}_3}$). When these two compounds reacts with each other they form an adduct having the following structure
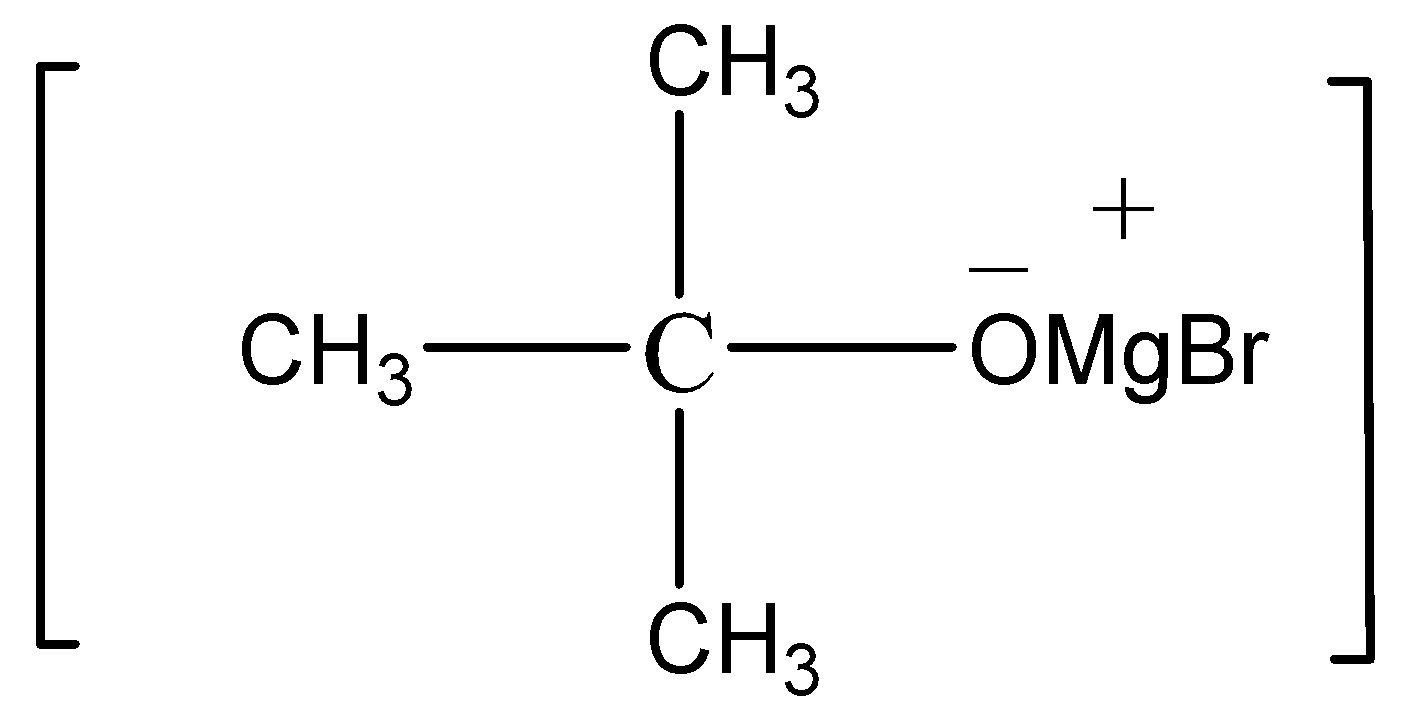
After the formation of adduct we add water to the adduct, The water reacts with adduct results in the formation of Mg(OH)-Br and 2-methyl-propan-2-ol.
The balanced equation is given down below
${\text{C}}{{\text{H}}_3} - {\text{CO}} - {\text{ C}}{{\text{H}}_3}{\text{ + C}}{{\text{H}}_3} - {\text{MgBr }} \to {\text{ Adduct }} \to {\text{ Mg(OH)}} - {\text{Br + }}2 - methyl - propan - 2 - ol.$
Note:
We must remember that an Adduct is a direct product of a reaction which contains all the atoms of the reactants. When an adduct is being formed no other compound is being formed alongside an adduct which means that a single product is being achieved. An example of adduct is the addition of sodium bisulphate to an aldehyde which produces a sulphonate. Sulfonate contains both the aldehyde and sodium bisulphate.
Complete step by step answer:
We must know in the laboratory, Grignard reagent plays a major role in the synthesis of aldehydes from alcohol. In Grignard reagent, Mg metal is bonded with alkyl or aryl group by highly polar covalent bond and Mg bonded with halogen by ionic bond. Examples of Grignard reagents are methyl magnesium chloride $\left( {C{H_3}MgCl} \right)$, methyl magnesium bromide $\left( {C{H_3}MgBr} \right)$and phenyl magnesium bromide $\left( {{C_6}{H_5}MgBr} \right)$ which are collectively called as organo magnesium compound.
For the preparation of 2-methyl-propan-2-ol from methyl magnesium bromide, first we react the methyl magnesium bromide with propanone (having molecular formula${\text{C}}{{\text{H}}_3} - {\text{CO}} - {\text{C}}{{\text{H}}_3}$). When these two compounds reacts with each other they form an adduct having the following structure

After the formation of adduct we add water to the adduct, The water reacts with adduct results in the formation of Mg(OH)-Br and 2-methyl-propan-2-ol.
The balanced equation is given down below
${\text{C}}{{\text{H}}_3} - {\text{CO}} - {\text{ C}}{{\text{H}}_3}{\text{ + C}}{{\text{H}}_3} - {\text{MgBr }} \to {\text{ Adduct }} \to {\text{ Mg(OH)}} - {\text{Br + }}2 - methyl - propan - 2 - ol.$
Note:
We must remember that an Adduct is a direct product of a reaction which contains all the atoms of the reactants. When an adduct is being formed no other compound is being formed alongside an adduct which means that a single product is being achieved. An example of adduct is the addition of sodium bisulphate to an aldehyde which produces a sulphonate. Sulfonate contains both the aldehyde and sodium bisulphate.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




