
How will you prepare benzaldehyde from toluene and benzene.
Answer
573.6k+ views
Hint: Benzaldehyde is a colourless liquid, and steam volatile. It is found to be sparingly soluble in water but is very soluble inorganic solvents. As we know that conversion of toluene or benzene to Benzaldehyde is nowadays used as a laboratory method to produce Benzaldehyde.
Complete answer:
- Let’s first discuss about how Benzaldehyde can be prepared from toluene:
As we know that toluene can be converted to Benzaldehyde by a reaction called Etard reaction. We can see that in this reaction Benzaldehyde is formed by the oxidation of toluene with chromic oxide in acetic anhydride.
Basically, Benzaldehyde is found to react with the acetic anhydride to form benzylidene diacetate. This on further hydrolysis with acid or with an alkali produces Benzaldehyde. We can write the reaction as:
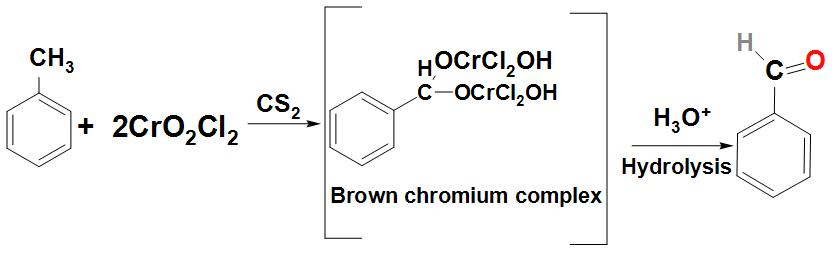
- Let’s now discuss about how Benzaldehyde can be prepared from benzene:
Benzaldehyde can be prepared from benzene by passing the vapours of HCl and CO in its solution. This reaction is found to take place in the presence of a mixture of catalyst $AlC{{l}_{3}}$ and CuCl. This reaction is called the Gatterman Koch reaction. We can write the reaction as:
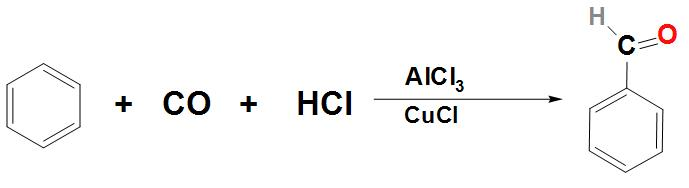
Note: - As we know that, Benzaldehyde is mostly used in industries. Benzaldehyde is actually an organic compound that basically consists of a benzene ring. It is found to contain a formyl substituent.
- It is also found to be less reactive than that of aliphatic aldehyde.
Complete answer:
- Let’s first discuss about how Benzaldehyde can be prepared from toluene:
As we know that toluene can be converted to Benzaldehyde by a reaction called Etard reaction. We can see that in this reaction Benzaldehyde is formed by the oxidation of toluene with chromic oxide in acetic anhydride.
Basically, Benzaldehyde is found to react with the acetic anhydride to form benzylidene diacetate. This on further hydrolysis with acid or with an alkali produces Benzaldehyde. We can write the reaction as:

- Let’s now discuss about how Benzaldehyde can be prepared from benzene:
Benzaldehyde can be prepared from benzene by passing the vapours of HCl and CO in its solution. This reaction is found to take place in the presence of a mixture of catalyst $AlC{{l}_{3}}$ and CuCl. This reaction is called the Gatterman Koch reaction. We can write the reaction as:

Note: - As we know that, Benzaldehyde is mostly used in industries. Benzaldehyde is actually an organic compound that basically consists of a benzene ring. It is found to contain a formyl substituent.
- It is also found to be less reactive than that of aliphatic aldehyde.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




