
Product A in the given reaction is:
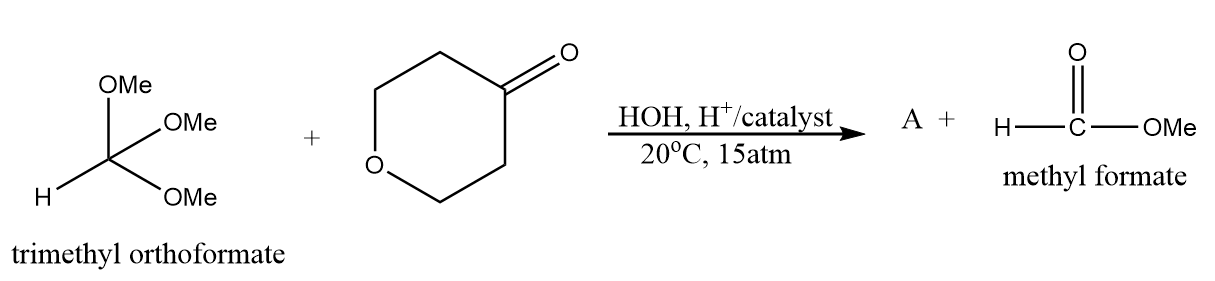
a.
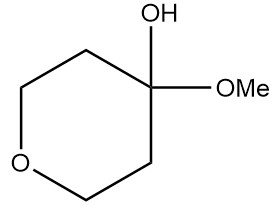
b.
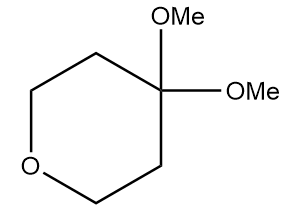
c.
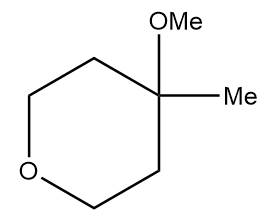
d.





Answer
513k+ views
Hint: In organic chemistry, an orthoester is a function group which contains three alkoxy groups and has a general formula of $RC{(OR')_3}$. These functional groups readily undergo hydrolysis reactions in the presence of mild aqueous acid to generate esters or respective carboxylic acids.
Complete answer: In the given reaction sequence, trimethyl orthoformate consists of three methoxy groups due to which the compound is very unstable. So, when it is exposed to water molecules in the acidic medium then instead of reacting it with given ketone, hydrolysis of the trimethyl orthoformate will take place. The reaction mechanism for the process is as follows:
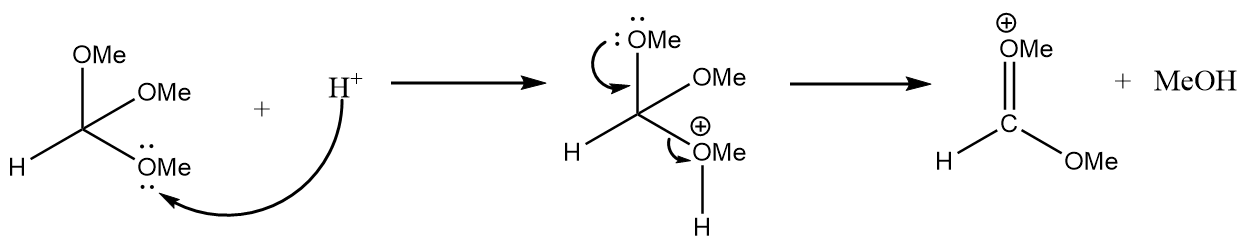
Step-1: Lone pair of electrons of methoxy group will attack the hydrogen ion and formation of an unstable intermediate takes place along with the removal of methanol. The reaction is as follows:
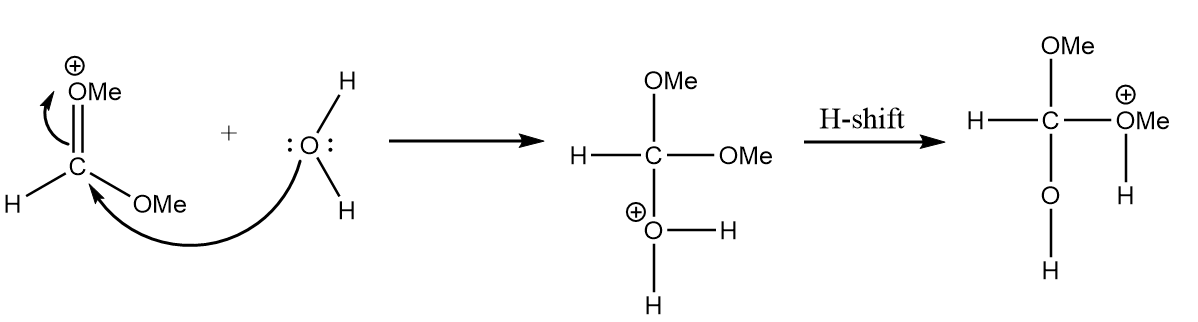
Step-2: As the positive charge on the oxygen atom makes the molecule unstable, so the attack of water molecules on electron deficient carbon will take place. The reaction is as follows:
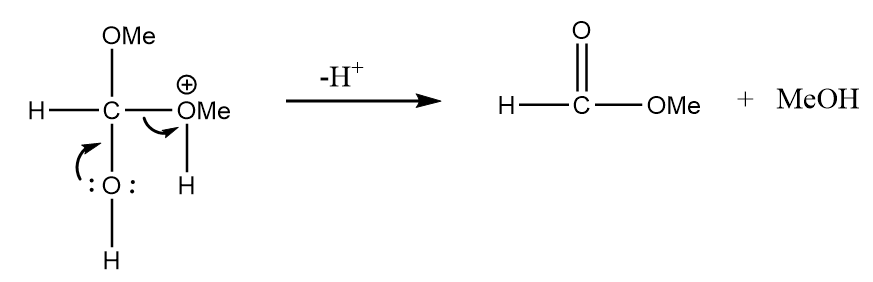
Step-3: The hydrogen ion will be released and methyl formate will be formed along with the removal of methyl alcohol. The reaction is as follows:
After the hydrolysis reaction of trimethyl orthoformate, two moles of methyl alcohol are formed which react with the given ketone to give the product A. The reaction mechanism for the process is as follows:
Step-1: Lone pair of electrons of oxygen atom of ketone group attack hydrogen ion and formation of carbocation takes place. The reaction is as follows:
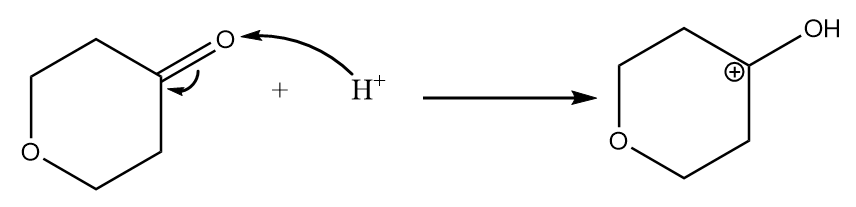
Step-2: Attack of methyl alcohol on carbocation takes place and the final product is formed along with the removal of hydrogen ions.
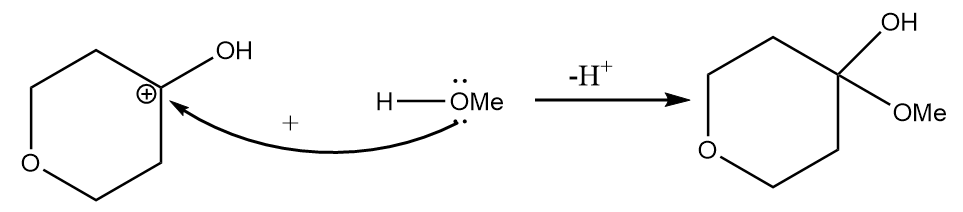
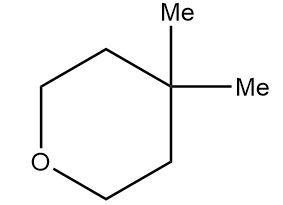
Hence, the structure of product A formed after the reaction is as follows:
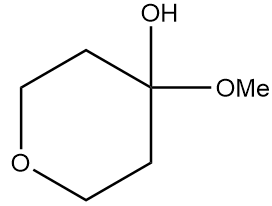
Therefore, option (A) is the correct answer.
Note:
It is important to note that ${H^ + }$ ion here represents the acidic medium which is generally ${H_2}S{O_4}$ in most of the reactions. Moreover, in the reaction sequence, the ${H^ + }$ ion acts as a catalyst i.e., none of the mole of hydrogen ion is consumed in the final product.
Complete answer: In the given reaction sequence, trimethyl orthoformate consists of three methoxy groups due to which the compound is very unstable. So, when it is exposed to water molecules in the acidic medium then instead of reacting it with given ketone, hydrolysis of the trimethyl orthoformate will take place. The reaction mechanism for the process is as follows:
Step-1: Lone pair of electrons of methoxy group will attack the hydrogen ion and formation of an unstable intermediate takes place along with the removal of methanol. The reaction is as follows:

Step-2: As the positive charge on the oxygen atom makes the molecule unstable, so the attack of water molecules on electron deficient carbon will take place. The reaction is as follows:

Step-3: The hydrogen ion will be released and methyl formate will be formed along with the removal of methyl alcohol. The reaction is as follows:

After the hydrolysis reaction of trimethyl orthoformate, two moles of methyl alcohol are formed which react with the given ketone to give the product A. The reaction mechanism for the process is as follows:
Step-1: Lone pair of electrons of oxygen atom of ketone group attack hydrogen ion and formation of carbocation takes place. The reaction is as follows:

Step-2: Attack of methyl alcohol on carbocation takes place and the final product is formed along with the removal of hydrogen ions.

Hence, the structure of product A formed after the reaction is as follows:

Therefore, option (A) is the correct answer.
Note:
It is important to note that ${H^ + }$ ion here represents the acidic medium which is generally ${H_2}S{O_4}$ in most of the reactions. Moreover, in the reaction sequence, the ${H^ + }$ ion acts as a catalyst i.e., none of the mole of hydrogen ion is consumed in the final product.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




