
\[Propanoic\;acid \xrightarrow[H_2O]{Cl_2/Red\;P} {\rm{X}}\].
What is \[{\rm{X}}\]?
A) Propanal
B) Propanol
C) propane
D) \[\alpha {\rm{ - }}\] chloro propanoic acid
Answer
584.1k+ views
Hint: We know that the reaction of carboxylic acid which must have \[\alpha {\rm{ - }}\] hydrogen atom with bromine or chlorine results in the formation of \[{\rm{2 - }}\] bromo carboxylic acid or \[{\rm{2 - }}\] chloro carboxylic acid in the presence of red phosphorus. The halogenation takes place during the reaction at the \[\alpha {\rm{ - }}\] carbon atom.
Complete answer:
This reaction is carried out on the basis of Hell - Volhard - Zelinsky halogenation reaction halogenated carboxylic acids at the \[\alpha\] carbon atom.
We know that propanoic acid is the carboxylic acid with \[\alpha {\rm{ - }}\] hydrogen atom. The reaction of propanoic acid with chlorine in the presence of red phosphorus leads to the formation of \[{\rm{2 - }}\]chloropropanoic acid which is also named as \[\alpha {\rm{ - }}\]chloro propanoic acid because the chlorination will take place at \[\alpha {\rm{ - }}\]carbon atom.
We can write the chemical equation for the reaction carried as follows.
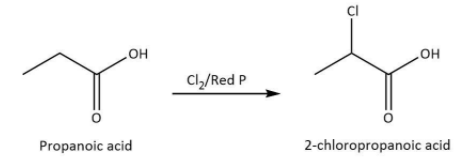
Hence, we can conclude that the correct option is D.
Note: The reaction which is carried out in the above question is commonly known as Hell-Volhard-Zelinsky reaction. The reaction takes place by the formation of phosphorous trichloride as the in-situ which catalysis the reaction. Therefore, we can say that the reaction is acid catalyzed enolization which is followed by the chlorination at alpha position.
Complete answer:
This reaction is carried out on the basis of Hell - Volhard - Zelinsky halogenation reaction halogenated carboxylic acids at the \[\alpha\] carbon atom.
We know that propanoic acid is the carboxylic acid with \[\alpha {\rm{ - }}\] hydrogen atom. The reaction of propanoic acid with chlorine in the presence of red phosphorus leads to the formation of \[{\rm{2 - }}\]chloropropanoic acid which is also named as \[\alpha {\rm{ - }}\]chloro propanoic acid because the chlorination will take place at \[\alpha {\rm{ - }}\]carbon atom.
We can write the chemical equation for the reaction carried as follows.

Hence, we can conclude that the correct option is D.
Note: The reaction which is carried out in the above question is commonly known as Hell-Volhard-Zelinsky reaction. The reaction takes place by the formation of phosphorous trichloride as the in-situ which catalysis the reaction. Therefore, we can say that the reaction is acid catalyzed enolization which is followed by the chlorination at alpha position.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




