
Propyl amine and aniline can be distinguished by azo dye test.
A. True
B. False
Answer
568.8k+ views
Hint: We should know what azo dye test and for what it is used. The azo dye test is used to distinguish amines. The amines are distinguished based on the formation of azo compounds. The azo compounds show colours.
Complete Answer :
The functional group amine is represented as:
$ - {\text{N}}{{\text{H}}_{\text{2}}}$. The ${\text{R}} - {\text{N}}{{\text{H}}_2}$is known as an amine. Here, R can be alkyl then the amine is known as aliphatic amine and if the R is aryl group then the amine is known as an aromatic amine.
On the basis of attachments amine are of three types:
Primary amine having $ - {\text{N}}{{\text{H}}_2}$ group.
Secondary amine having $ - {\text{NH}}\, - $ group.
Tertiary amine having $ - \mathop {\text{N}}\limits_| - $ group.
The primary, secondary and tertiary amine have different chemical and physical properties and hence different reactivates.
So, different tests are used to differentiate the primary, secondary and tertiary amines.
The azo dye test is used to distinguish aromatic and aliphatic amines.
In this test, amines are reacted with nitrous acid, so a diazonium salt forms. The ${{\text{N}}_{\text{2}}}$ of diazonium salt of aromatic amine act as an electrophile so, another aromatic amine attacks on this electrophile and ${{\text{N}}_{\text{2}}}$get bridged between two aromatic amines. The azo compounds show colour hence used as a dye. Thus the test is known as azo dye test.So, we will find the aromatic amine.
The structure of the given amines are as follows:
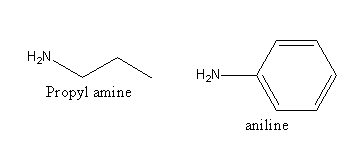
Propyl amine is aliphatic and aniline is an aromatic amine. So, aniline will form the azo compound and propylamine do not, so both can be distinguished by the azo dye test. In the diazo test, aniline is reacted with nitrous acid then beta-naphthol.
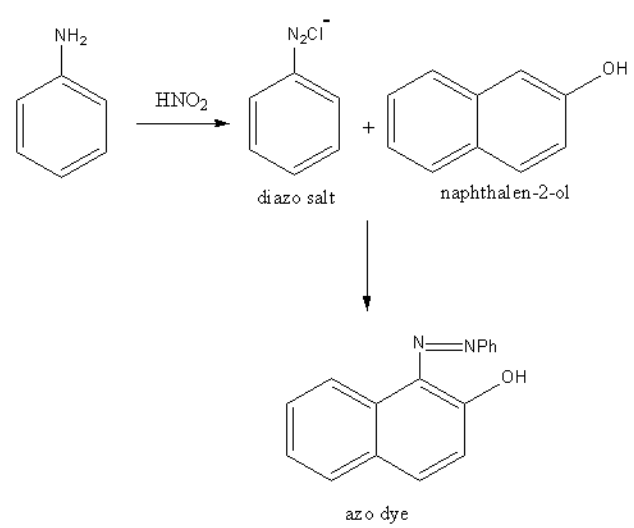
The formed azo dye has an orange colour.
So, it is true that propyl amine and aniline can be distinguished by the azo dye test.
Therefore, option (A) true, is correct.
Note: The test used to differentiate amine are the carbylamine test, nitrous acid test, solubility, and litmus test, Hinsberg, and azo dye test. Litmus and solubility tests are used to determine the presence of an amine. Carbylamine, nitrous acid and Hinsberg test are used to differentiate primary, secondary and tertiary amines. An azo dye test is used to differentiate aliphatic and aromatic amines.
Complete Answer :
The functional group amine is represented as:
$ - {\text{N}}{{\text{H}}_{\text{2}}}$. The ${\text{R}} - {\text{N}}{{\text{H}}_2}$is known as an amine. Here, R can be alkyl then the amine is known as aliphatic amine and if the R is aryl group then the amine is known as an aromatic amine.
On the basis of attachments amine are of three types:
Primary amine having $ - {\text{N}}{{\text{H}}_2}$ group.
Secondary amine having $ - {\text{NH}}\, - $ group.
Tertiary amine having $ - \mathop {\text{N}}\limits_| - $ group.
The primary, secondary and tertiary amine have different chemical and physical properties and hence different reactivates.
So, different tests are used to differentiate the primary, secondary and tertiary amines.
The azo dye test is used to distinguish aromatic and aliphatic amines.
In this test, amines are reacted with nitrous acid, so a diazonium salt forms. The ${{\text{N}}_{\text{2}}}$ of diazonium salt of aromatic amine act as an electrophile so, another aromatic amine attacks on this electrophile and ${{\text{N}}_{\text{2}}}$get bridged between two aromatic amines. The azo compounds show colour hence used as a dye. Thus the test is known as azo dye test.So, we will find the aromatic amine.
The structure of the given amines are as follows:

Propyl amine is aliphatic and aniline is an aromatic amine. So, aniline will form the azo compound and propylamine do not, so both can be distinguished by the azo dye test. In the diazo test, aniline is reacted with nitrous acid then beta-naphthol.

The formed azo dye has an orange colour.
So, it is true that propyl amine and aniline can be distinguished by the azo dye test.
Therefore, option (A) true, is correct.
Note: The test used to differentiate amine are the carbylamine test, nitrous acid test, solubility, and litmus test, Hinsberg, and azo dye test. Litmus and solubility tests are used to determine the presence of an amine. Carbylamine, nitrous acid and Hinsberg test are used to differentiate primary, secondary and tertiary amines. An azo dye test is used to differentiate aliphatic and aromatic amines.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




