
PVC is the polymer of
Answer
516.6k+ views
Hint: The repeated units of monomers i.e. compounds formed by the combination of many simple molecules are called polymers. Based upon molecular forces polymers are classified: elastomers, fibers, thermoplastic and thermosetting polymers.
Thermoplastic Polymers gets easily soft when heated
The force between the molecules is between elastomers and fibers and is usually formed by addition polymerization. Examples: PVC, Teflon
Complete answer:
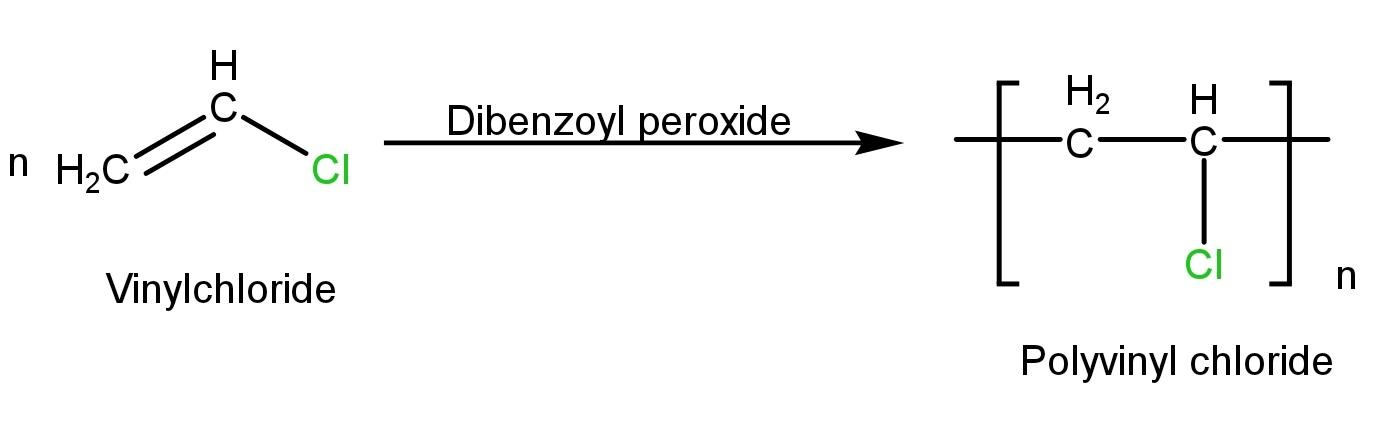
PVC is a thermoplastic polymer; the monomer of PVC is Vinyl chloride. The formation of PVC polymer occurs by an addition reaction in the addition reaction the monomers combine directly with each other without the formation of by-products such as water. Vinyl chloride is an unsaturated molecule and unsaturated molecules generally chain-growth polymers. PVC softens and can be molded easily because there is no cross-linkage between the polymer chains. The structure of PVC is linear and it is a homopolymer which means PVC has only one kind of monomeric unit which is Vinyl chloride.
PVC is synthesized by addition reactions of a large number of vinyl chloride molecules-
Note:
We often get confused between Polymers and macromolecules. All macromolecules and not polymers but all polymers are macromolecules one of the best examples of this is chlorophyll which is not a polymer but is a macromolecule.
PVC has good insulating properties. They are mainly used in the formation of pipes. Due to their attractive properties of low maintenance cost and less weight. They are also used in the manufacturing of leather coats and raincoats.
Thermoplastic Polymers gets easily soft when heated
The force between the molecules is between elastomers and fibers and is usually formed by addition polymerization. Examples: PVC, Teflon
Complete answer:
PVC is a thermoplastic polymer; the monomer of PVC is Vinyl chloride. The formation of PVC polymer occurs by an addition reaction in the addition reaction the monomers combine directly with each other without the formation of by-products such as water. Vinyl chloride is an unsaturated molecule and unsaturated molecules generally chain-growth polymers. PVC softens and can be molded easily because there is no cross-linkage between the polymer chains. The structure of PVC is linear and it is a homopolymer which means PVC has only one kind of monomeric unit which is Vinyl chloride.

PVC is synthesized by addition reactions of a large number of vinyl chloride molecules-

Note:
We often get confused between Polymers and macromolecules. All macromolecules and not polymers but all polymers are macromolecules one of the best examples of this is chlorophyll which is not a polymer but is a macromolecule.
PVC has good insulating properties. They are mainly used in the formation of pipes. Due to their attractive properties of low maintenance cost and less weight. They are also used in the manufacturing of leather coats and raincoats.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




