
Reaction of aniline with acetyl chloride in the presence of \[{\rm{NaOH}}\] gives:
A) acetanilide
B) P-chloroaniline
C) a red eye
D) aniline hydrochloride
Answer
584.1k+ views
Hint: It is known that the nucleophilic reaction of the nitrogen of aromatic amines which bears a lone pair of electrons with haloalkanes and with the acid chlorides is regarded as the nucleophilic substitution reaction.
Complete answer:
Generally, we know that \[{{\rm{S}}_{\rm{N}}}1\] and \[{{\rm{S}}_{\rm{N}}}2\] are the mechanisms for the nucleophilic substitution reaction in which the bond breaking of the carbon with leaving group and the formation of a new bond with a nucleophile. \[{{\rm{S}}_{\rm{N}}}1\] mechanism is a unimolecular reaction where it involves one species in the rate defining step, whereas \[{{\rm{S}}_{\rm{N}}}2\] is a bimolecular reaction, it involves two species.
We know that aniline is the primary aromatic amine. The reaction in which aniline reacts with acid chlorides, the final product of the reaction will be N-substituted amine.
Therefore, when we treat aniline with acetyl chloride in the presence of \[{\rm{NaOH}}\], the formation of acetanilide takes place.
We can write the chemical equation for the reaction carried as follows.
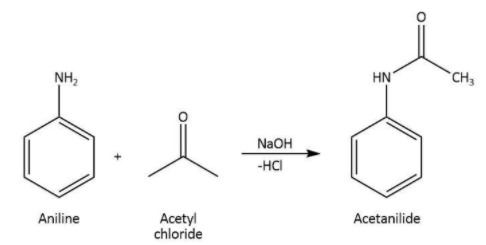
Hence, we can conclude that the correct option is A.
Note: It is known that the reaction of aromatic amine with aliphatic acid chloride or the aliphatic acid anhydride results in the formation of amides. The acetylation reaction is best carried out with acetic anhydride rather than acetyl chloride.
Complete answer:
Generally, we know that \[{{\rm{S}}_{\rm{N}}}1\] and \[{{\rm{S}}_{\rm{N}}}2\] are the mechanisms for the nucleophilic substitution reaction in which the bond breaking of the carbon with leaving group and the formation of a new bond with a nucleophile. \[{{\rm{S}}_{\rm{N}}}1\] mechanism is a unimolecular reaction where it involves one species in the rate defining step, whereas \[{{\rm{S}}_{\rm{N}}}2\] is a bimolecular reaction, it involves two species.
We know that aniline is the primary aromatic amine. The reaction in which aniline reacts with acid chlorides, the final product of the reaction will be N-substituted amine.
Therefore, when we treat aniline with acetyl chloride in the presence of \[{\rm{NaOH}}\], the formation of acetanilide takes place.
We can write the chemical equation for the reaction carried as follows.

Hence, we can conclude that the correct option is A.
Note: It is known that the reaction of aromatic amine with aliphatic acid chloride or the aliphatic acid anhydride results in the formation of amides. The acetylation reaction is best carried out with acetic anhydride rather than acetyl chloride.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




