
Reaction of phenol with dilute $HN{O_3}$ gives
A. p- and m- nitrophenol
B. o- and p- nitrophenol
C. picric acid
D. o- and m- nitrophenol
Answer
513.3k+ views
Hint: Electrophilic aromatic substitution reaction: It is an organic reaction in which an electrophile replaces an atom attached to the aromatic ring. In general reaction, the hydrogen atom is replaced by an electrophile in a benzene ring. Examples of these reactions are nitration, halogenation, Friedel craft alkylation, etc.
Complete answer: As the hydroxyl group on phenol is ortho para directing, so when phenol reacts with dilute sulphuric acid, the attack of nitronium ion takes place at ortho and para positions of the benzene ring. The reaction mechanism involved in the process is as follows:
Step-1: Reaction of dilute nitric acid with catalytic hydrogen ion to form nitronium ion along with the removal of water molecules. The reaction proceeds as follows:
${H^ + } + HN{O_3} \to NO_2^ + + {H_2}O$
Step-2: Nitronium ion acts as an electrophile and attacks at the ortho position of the phenol to form ortho nitrophenol. The reaction takes place as follows:
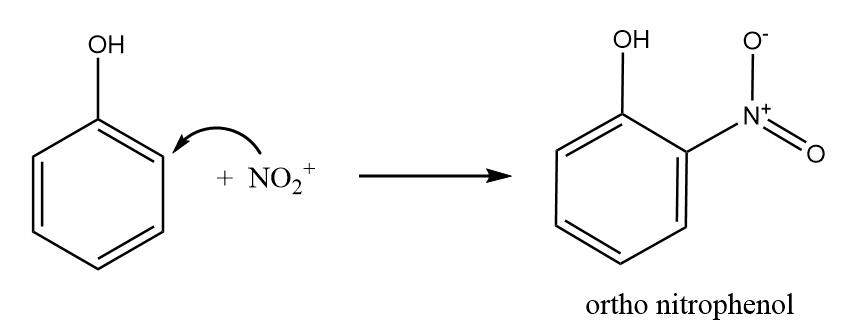
Step-3: Nitronium ion acts as an electrophile and attacks at the para position of the phenol to form para nitrophenol. The reaction takes place as follows:
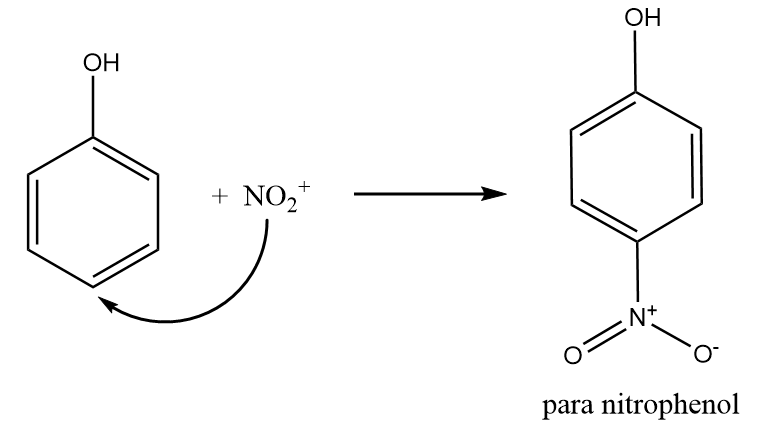
Hence, the reaction of phenol with dilute $HN{O_3}$ gives o- and p- nitrophenol. Therefore, option (B) is the right answer.
Note:
It is important to note that when phenol reacts with concentrated nitric acid, then instead of formation of ortho and para nitrophenol, formation of picric acid takes place under such conditions. The reaction proceeds as follows:

Complete answer: As the hydroxyl group on phenol is ortho para directing, so when phenol reacts with dilute sulphuric acid, the attack of nitronium ion takes place at ortho and para positions of the benzene ring. The reaction mechanism involved in the process is as follows:
Step-1: Reaction of dilute nitric acid with catalytic hydrogen ion to form nitronium ion along with the removal of water molecules. The reaction proceeds as follows:
${H^ + } + HN{O_3} \to NO_2^ + + {H_2}O$
Step-2: Nitronium ion acts as an electrophile and attacks at the ortho position of the phenol to form ortho nitrophenol. The reaction takes place as follows:

Step-3: Nitronium ion acts as an electrophile and attacks at the para position of the phenol to form para nitrophenol. The reaction takes place as follows:

Hence, the reaction of phenol with dilute $HN{O_3}$ gives o- and p- nitrophenol. Therefore, option (B) is the right answer.
Note:
It is important to note that when phenol reacts with concentrated nitric acid, then instead of formation of ortho and para nitrophenol, formation of picric acid takes place under such conditions. The reaction proceeds as follows:

Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




