
Reduction of \[cyclohex-2-enone\] with aluminium isopropoxide more than isopropyl alcohol will produce:
A.Cyclohexanol
B.\[Cyclohex-2-enol\]
C.Cyclohexanone
D.Benzene
Answer
509.1k+ views
Hint: We know that the oppenauer Oxidation is a chemical conversion process in which, the secondary alcohols that are present in each composition turn to ketones under a controlled atmosphere with the help of selective oxidation.
Complete answer:
As we know that the Meerwein-Ponndorf reaction: In organic chemistry, this reaction is actually named as Meerwein-Ponndorf-Verley or MPV reduction is the reduction of ketones and aldehydes to alcohols using aluminium alkoxide catalysts in the presence of a sacrificial alcohol. This reaction is called MPV (Meerwein–Ponndorf–Verley reduction) reaction; the reverse of it is called oppenauer oxidation reaction. Side product is ketone.
\[2R-C=O\xrightarrow[C{{H}_{3}}-CH(OH)-C{{H}_{3}}]{\underset{Aluminium\text{ }Isopropoxide}{\mathop{{{[C{{H}_{3}}C-O]}_{3}}Al}}\,}\underset{M.P.V.\text{ }Reduction}{\mathop{2R-CH-OH}}\,\]
Oppenauer oxidation is the process where an aluminium alkoxide has catalyzed oxidation of the secondary alcohol that is present over the corresponding ketone. The oppenauer oxidation is the reverse process of Meerwein Ponndorf Verley reduction. The oppenauer oxidation reaction is one of the most reliable methods that help oxidize allylic alcohols to \[\alpha ,\text{ }\beta -\] unsaturated ketones. The oxidation reaction comes as an exact result of the reverse of Meerwein-Ponndorf-Verley reduction. It involves the process of deprotonation of the alcohol by equilibration over the alkoxide, concluded by a hydride transfer.
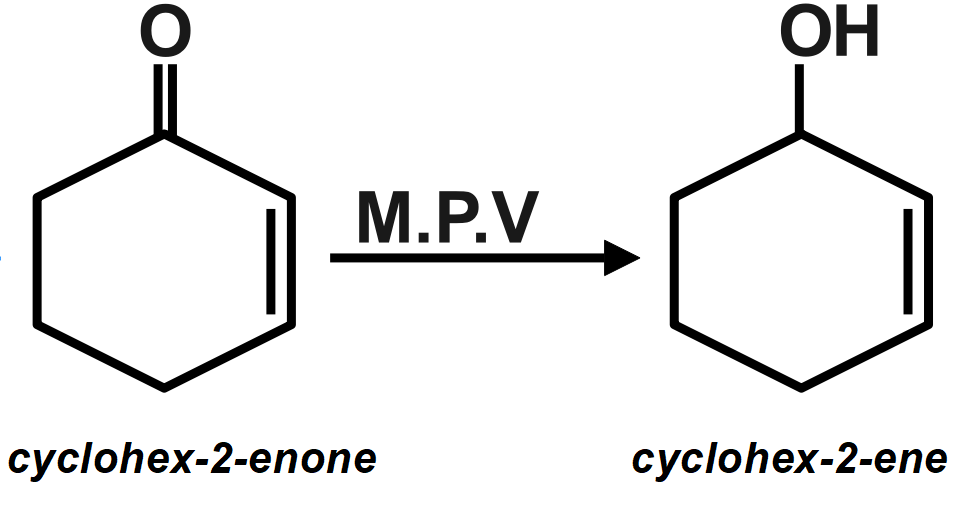
Therefore, the correct answer is option B.
Note:
Remember that the traditional Oppenauer Oxidation is a highly chemo selective oxidation process. However, the reactions and its mechanism do come with their own set of disadvantages. To begin with, the reaction method makes use of high temperatures that is generally achieved using large quantities of ketone hydride acceptors.
Complete answer:
As we know that the Meerwein-Ponndorf reaction: In organic chemistry, this reaction is actually named as Meerwein-Ponndorf-Verley or MPV reduction is the reduction of ketones and aldehydes to alcohols using aluminium alkoxide catalysts in the presence of a sacrificial alcohol. This reaction is called MPV (Meerwein–Ponndorf–Verley reduction) reaction; the reverse of it is called oppenauer oxidation reaction. Side product is ketone.
\[2R-C=O\xrightarrow[C{{H}_{3}}-CH(OH)-C{{H}_{3}}]{\underset{Aluminium\text{ }Isopropoxide}{\mathop{{{[C{{H}_{3}}C-O]}_{3}}Al}}\,}\underset{M.P.V.\text{ }Reduction}{\mathop{2R-CH-OH}}\,\]
Oppenauer oxidation is the process where an aluminium alkoxide has catalyzed oxidation of the secondary alcohol that is present over the corresponding ketone. The oppenauer oxidation is the reverse process of Meerwein Ponndorf Verley reduction. The oppenauer oxidation reaction is one of the most reliable methods that help oxidize allylic alcohols to \[\alpha ,\text{ }\beta -\] unsaturated ketones. The oxidation reaction comes as an exact result of the reverse of Meerwein-Ponndorf-Verley reduction. It involves the process of deprotonation of the alcohol by equilibration over the alkoxide, concluded by a hydride transfer.

Therefore, the correct answer is option B.
Note:
Remember that the traditional Oppenauer Oxidation is a highly chemo selective oxidation process. However, the reactions and its mechanism do come with their own set of disadvantages. To begin with, the reaction method makes use of high temperatures that is generally achieved using large quantities of ketone hydride acceptors.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




