
What is refining of metals? Explain with diagram the method of electrolysis by
Which copper is purified?
Answer
596.1k+ views
Hint: Copper is widely used in electrical industries for making wiring due to its high electrical conductivity. But for this purpose, a purity of more than 99.7% is required. This purification of copper is achieved by electrorefining.
Complete step by step answer:
Refining of metal refers to the process in which impure metals are purified. It is of various types. Electrolysis of copper is an example of electrolytic refining.
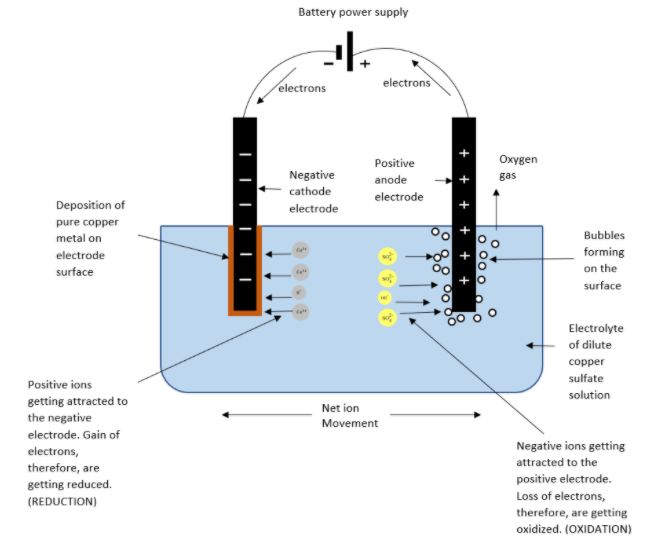
When copper is extracted from its sulphide ores, it remains impure. To make it pure, a process called electrolysis is used. In this process, a pure copper sheet/rod and an impure copper/rod sheet is immersed in an electrolytic solution made up of copper (II) sulfate. The impure one acts as anode while the pure on as cathode. When a dc electric current is passed through the solution, copper from the positively charged anode gets deposited on the negatively charged cathode, while the impurities go into the \[CuS{{O}_{4}}\] solution called as anode mud.
Chemical reaction:
At cathode
\[C{{u}^{2+}}\,+\,2e\,\to \,Cu\]
At anode:
\[Cu\,-\,2e\,\to \,C{{u}^{2+}}\]
Note: Copper ores occur in different forms and the extraction method of copper depends on the nature of the ore. Sulphide ores such as chalcopyrite are converted to copper by a different method as compared to silicate, carbonate or sulphate ores.
Complete step by step answer:
Refining of metal refers to the process in which impure metals are purified. It is of various types. Electrolysis of copper is an example of electrolytic refining.
When copper is extracted from its sulphide ores, it remains impure. To make it pure, a process called electrolysis is used. In this process, a pure copper sheet/rod and an impure copper/rod sheet is immersed in an electrolytic solution made up of copper (II) sulfate. The impure one acts as anode while the pure on as cathode. When a dc electric current is passed through the solution, copper from the positively charged anode gets deposited on the negatively charged cathode, while the impurities go into the \[CuS{{O}_{4}}\] solution called as anode mud.
Chemical reaction:
At cathode
\[C{{u}^{2+}}\,+\,2e\,\to \,Cu\]
At anode:
\[Cu\,-\,2e\,\to \,C{{u}^{2+}}\]

Note: Copper ores occur in different forms and the extraction method of copper depends on the nature of the ore. Sulphide ores such as chalcopyrite are converted to copper by a different method as compared to silicate, carbonate or sulphate ores.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




