
What is the relationship between the structures shown?
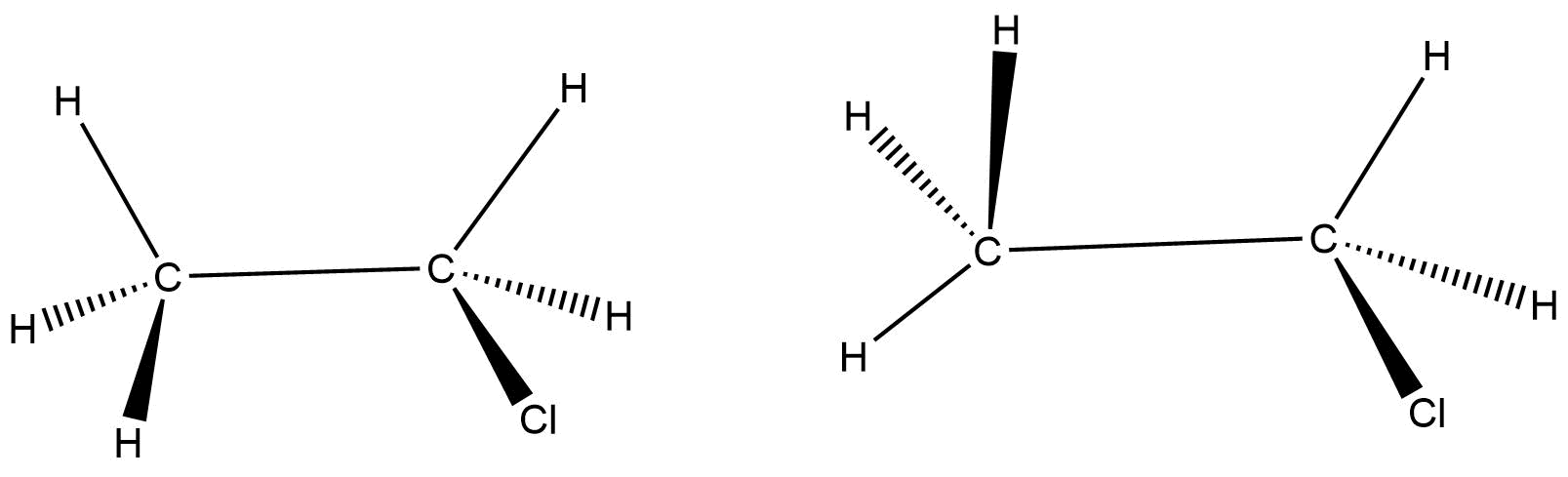
(A) Structural isomers
(B) Geometrical isomers
(C) Conformational structures
(D) Identical structures
Answer
233.1k+ views
Hint: Isomers are the same molecules with different spatial arrangements of their atoms or having different bonding patterns. Depending upon the different arrangements, the isomers are classified and we can identify the type of isomer by spotting the differences between two molecules.
Complete answer:
In order to find out the actual relation between the mentioned molecules, let us mention isomers.
What are isomers?
In chemistry, isomers are defined as the molecules that possess the same molecular formula, but their atoms have different spatial arrangements.
A prime example of isomers is n-butane and isobutane. They both have the same molecular formula, i.e., ${C_4}{H_{10}}$, but they differ due to the different arrangement of the methyl group.

Now, there are different isomers available in chemistry based on a number of different factors.
Let’s now define the isomers mentioned in the answer.
Structural isomers:
Structural isomers are those isomers which have the same molecular formula, but they have different bonding patterns or atomic organization.
For example: butane and isobutane. They have the same molecular formula, but the bonding patterns are different in both the molecules (the carbon chain is different in both molecules). The picture of both the molecules have been shown above.
Geometrical isomers
Geometrical isomers can be understood by the situation in which the two atoms (let’s say carbon atoms) are having a double bond and the bond rotation is restricted. Based on the position of the atoms attached to those carbon atoms will produce geometrical isomers. These isomers are also known as cis/trans isomers.
For example: trans-1,2-dichloroethelene and cis-1,2-dichloroethelene. Here, both the carbon atoms are attached to each other with a double bond. So, the bond rotation is restricted. Now, based on the position of chlorine atoms and hydrogen atoms, two geometrical isomers have been proposed.
In trans-1,2-dichloroethelene, two chlorine and hydrogen atoms are opposite to each other. In cis-1,2-dichloroethelene, both the two hydrogen and chlorine atoms are in the same side.
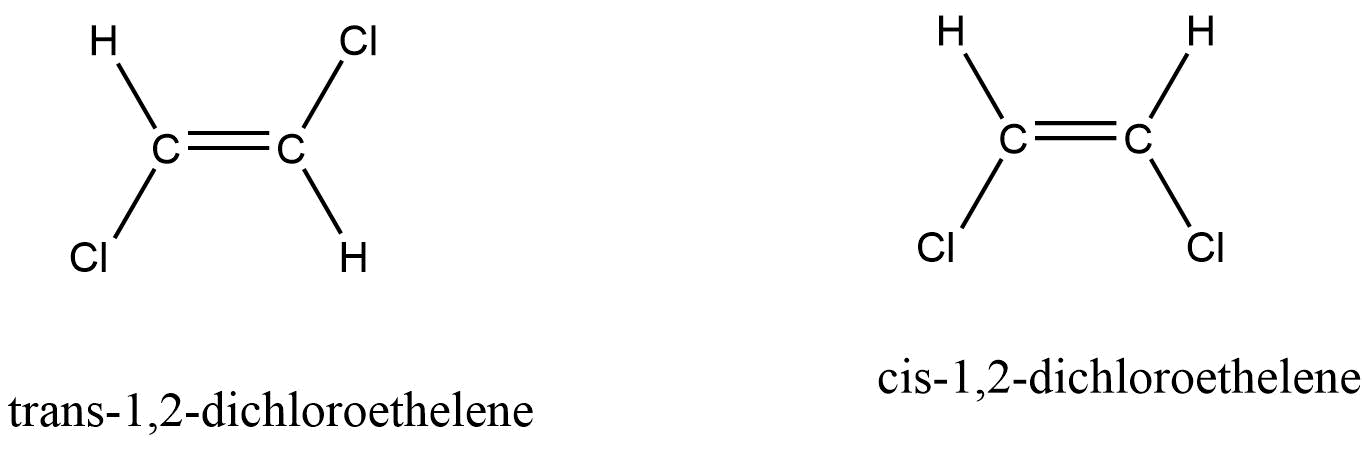
Conformational structures
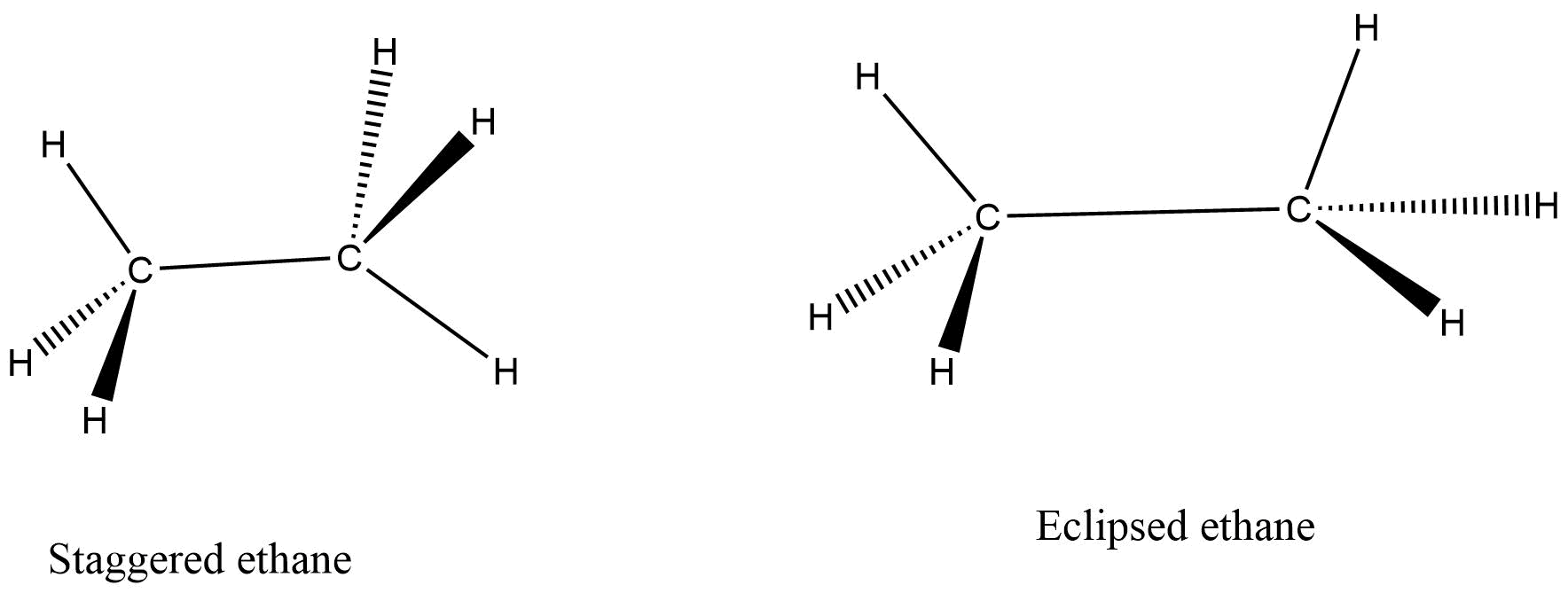
These structures are particularly obtained by rotation of single bonds.
When we rotate carbon – carbon single bond in a molecule, then a number of different conformers are created. The conformational structures are the stereoisomers and they are always interconvertible at room temperature.
Identical structures
When two molecules are absolutely the same, having the same spatial arrangement of their atoms and their bonding patterns are also same, then they are called identical structures.
Now, if we look at the molecule pair given in the question, then we will find that in both the molecules, the chlorine atom is above the plane. In the first molecule, the two hydrogen atoms (those who are in the plane of carbon atoms) are on the same side. One the other hand, in the second molecule, those two hydrogens are on the opposite side.
The second structure can easily be obtained if we rotate the carbon – carbon single bond of the first molecule and vice-versa.
Thus, since either of the structures can easily be achieved from the other one by just the rotation of a single bond, we can say these two structures are nothing but conformers. In other words, these two are conformational isomers or structures.
Hence, these two molecules are conformational structures.
Hence, option C is the correct answer to this question.
Note: Students many times get confused with different types of isomers. They have to remember, isomers are the same molecules, but they have different arrangements of their atoms or they are having different bonding structures. Hence, to determine the type of isomer correctly, students have to closely look at the arrangements of the atoms in the given molecules and find the differences.
Complete answer:
In order to find out the actual relation between the mentioned molecules, let us mention isomers.
What are isomers?
In chemistry, isomers are defined as the molecules that possess the same molecular formula, but their atoms have different spatial arrangements.
A prime example of isomers is n-butane and isobutane. They both have the same molecular formula, i.e., ${C_4}{H_{10}}$, but they differ due to the different arrangement of the methyl group.

Now, there are different isomers available in chemistry based on a number of different factors.
Let’s now define the isomers mentioned in the answer.
Structural isomers:
Structural isomers are those isomers which have the same molecular formula, but they have different bonding patterns or atomic organization.
For example: butane and isobutane. They have the same molecular formula, but the bonding patterns are different in both the molecules (the carbon chain is different in both molecules). The picture of both the molecules have been shown above.
Geometrical isomers
Geometrical isomers can be understood by the situation in which the two atoms (let’s say carbon atoms) are having a double bond and the bond rotation is restricted. Based on the position of the atoms attached to those carbon atoms will produce geometrical isomers. These isomers are also known as cis/trans isomers.
For example: trans-1,2-dichloroethelene and cis-1,2-dichloroethelene. Here, both the carbon atoms are attached to each other with a double bond. So, the bond rotation is restricted. Now, based on the position of chlorine atoms and hydrogen atoms, two geometrical isomers have been proposed.
In trans-1,2-dichloroethelene, two chlorine and hydrogen atoms are opposite to each other. In cis-1,2-dichloroethelene, both the two hydrogen and chlorine atoms are in the same side.
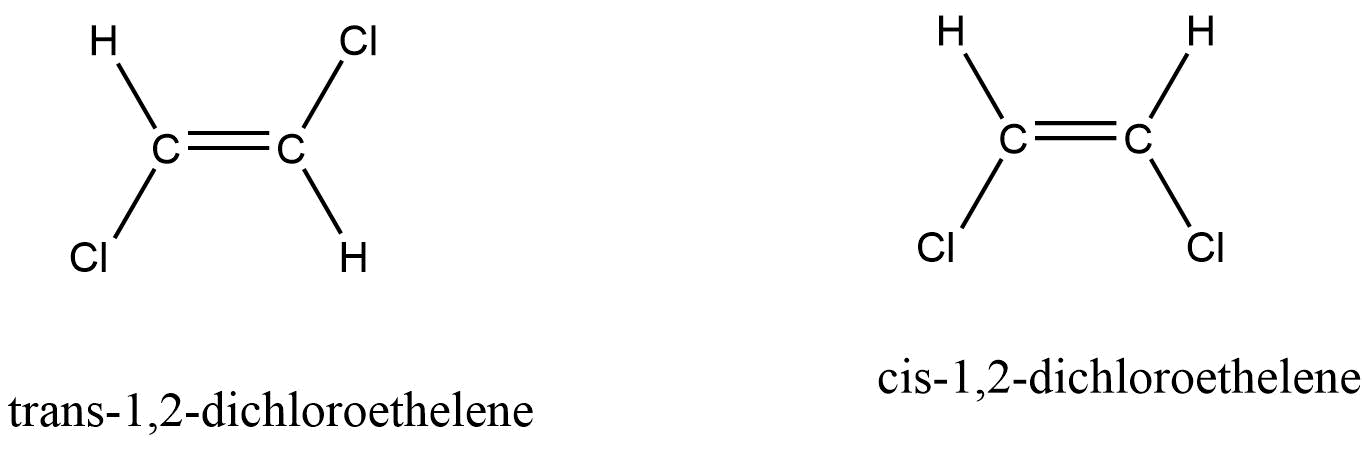
Conformational structures
These structures are particularly obtained by rotation of single bonds.
When we rotate carbon – carbon single bond in a molecule, then a number of different conformers are created. The conformational structures are the stereoisomers and they are always interconvertible at room temperature.

Identical structures
When two molecules are absolutely the same, having the same spatial arrangement of their atoms and their bonding patterns are also same, then they are called identical structures.
Now, if we look at the molecule pair given in the question, then we will find that in both the molecules, the chlorine atom is above the plane. In the first molecule, the two hydrogen atoms (those who are in the plane of carbon atoms) are on the same side. One the other hand, in the second molecule, those two hydrogens are on the opposite side.
The second structure can easily be obtained if we rotate the carbon – carbon single bond of the first molecule and vice-versa.
Thus, since either of the structures can easily be achieved from the other one by just the rotation of a single bond, we can say these two structures are nothing but conformers. In other words, these two are conformational isomers or structures.
Hence, these two molecules are conformational structures.
Hence, option C is the correct answer to this question.
Note: Students many times get confused with different types of isomers. They have to remember, isomers are the same molecules, but they have different arrangements of their atoms or they are having different bonding structures. Hence, to determine the type of isomer correctly, students have to closely look at the arrangements of the atoms in the given molecules and find the differences.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




