
How many \[S{\text{ }}-{\text{ }}0{\text{ }}-{\text{ }}S\] linkages are there in dithionous acid?
Answer
576.3k+ views
Hint: In oxyacid of sulfur, sulfur is the central atom exhibiting a tetrahedral structure when coordinated with oxygen. This contains at least one \[S = O\] bond and one \[S - OH\] bond. In addition to these, a chain of \[{( - S - )_n}\] as in \[{H_2}{S_2}{O_6}\] . Such oxyacids with \[S - S\] linkages are called Thioacids.
Complete step by step answer:
The sulfur oxyacids are the acids that contain oxygen, hydrogen, and sulfur. These are divided into four groups based on their structural similarities:
1.Sulphurous acid group- these are prepared by dissolving sulphur dioxide in water. Their structure is pyramidal with three oxygen atoms on a triangle i.e. tetrahedral structure is distorted by lone pair. These are strong reducing agents and have bleaching properties.
For example, sulphurous acid \[{H_2}S{O_3}\] .
2.Sulphuric acid group- these are also called oil of vitriol, produced by the lead chamber and contact processes by dissolving \[S{O_3}\] in water. For example, sulphuric acid \[{H_2}S{O_4}\] (oleum) and thiosulphuric acid.
3.Thionic acid group- these are a series of unstable acids with the general formula of \[{H_2}{S_n}{O_6}\] where n = 2 to 6. For example, Dithionic acid \[{H_2}{S_2}{O_6}\] .
4.Peroxo acid group- these are also called Marshall’s acid or Caro’s acid. The central atom sulphur has a +6-oxidation state with a peroxo group in it. These are derived from hydrogen peroxide by replacing hydrogen atoms. For example, peroxydisulfuric acid \[{H_2}{S_2}{O_8}\] .
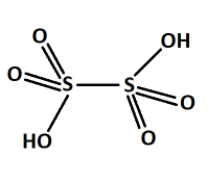
From all these series, we saw that the \[S - S\] bond is present only one in the thionic acid series. For example, dithionic acid \[{H_2}{S_2}{O_6}\] . The structure is shown below,
Note: All oxyacids have the acidic hydrogen bonded to an oxygen atom. i.e. \[ - OH\] bond. The electronegativity of the central atom and the number of O atoms are responsible for the oxyacid acidity.
Complete step by step answer:
The sulfur oxyacids are the acids that contain oxygen, hydrogen, and sulfur. These are divided into four groups based on their structural similarities:
1.Sulphurous acid group- these are prepared by dissolving sulphur dioxide in water. Their structure is pyramidal with three oxygen atoms on a triangle i.e. tetrahedral structure is distorted by lone pair. These are strong reducing agents and have bleaching properties.
For example, sulphurous acid \[{H_2}S{O_3}\] .
2.Sulphuric acid group- these are also called oil of vitriol, produced by the lead chamber and contact processes by dissolving \[S{O_3}\] in water. For example, sulphuric acid \[{H_2}S{O_4}\] (oleum) and thiosulphuric acid.
3.Thionic acid group- these are a series of unstable acids with the general formula of \[{H_2}{S_n}{O_6}\] where n = 2 to 6. For example, Dithionic acid \[{H_2}{S_2}{O_6}\] .
4.Peroxo acid group- these are also called Marshall’s acid or Caro’s acid. The central atom sulphur has a +6-oxidation state with a peroxo group in it. These are derived from hydrogen peroxide by replacing hydrogen atoms. For example, peroxydisulfuric acid \[{H_2}{S_2}{O_8}\] .
From all these series, we saw that the \[S - S\] bond is present only one in the thionic acid series. For example, dithionic acid \[{H_2}{S_2}{O_6}\] . The structure is shown below,

Note: All oxyacids have the acidic hydrogen bonded to an oxygen atom. i.e. \[ - OH\] bond. The electronegativity of the central atom and the number of O atoms are responsible for the oxyacid acidity.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




