
Separation of two immiscible liquids is based on:
(a) Difference in the solubility of the two liquids
(b) Difference in densities of the two liquids
(c) Difference in melting point of the two liquids
(d) Difference in boiling point of the two liquids
Answer
603k+ views
Hint: To answer this question, we must first clearly understand when and why are two liquids immiscible. We should also recall the concepts of separation of mixtures.
Complete step by step solution:
Immiscible liquids are those liquids which will not mix with each other to give a single phase. Oil and water are examples of immiscible liquids - one floats on top of the other. Though classified as immiscible, in actual fact, there is still some degree of mutual solubility. For example, in benzene and water, a small amount of benzene will be dissolved in the water phase, and vice versa.
Since by definition, immiscible liquids do not interact with each other in any way whatsoever, they will evaporate completely independent of each other.
For separating immiscible liquids like oil and water we use separating funnels.
The principle of separating funnels is that immiscible liquids separate out in layers depending on their densities.
Hence, the correct answer is Option (B) Difference in densities of the two liquids
Additional information:
We can separate a mixture of Kerosene oil and water in the following way.
1- Pour the mixture of kerosene oil and water in a separating funnel
2- Let it stand undisturbed for sometime so that separate layers of oil and water are formed.
3- Open the stopcock of the separating funnel and pour out the lower layer of water carefully.
4- Close the stopcock of the separating funnel as the oil reaches the stop-cock.
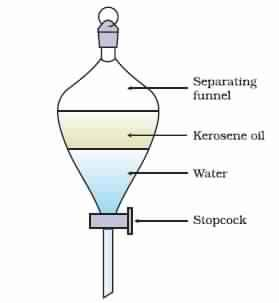
Note: This principle of separation of immiscible fluids on the basis of densities also finds application in the extraction of iron from its ore. The lighter kerosene oil is collected at the top of the separating funnel while heavier water will be pore out from the bottom.
Complete step by step solution:
Immiscible liquids are those liquids which will not mix with each other to give a single phase. Oil and water are examples of immiscible liquids - one floats on top of the other. Though classified as immiscible, in actual fact, there is still some degree of mutual solubility. For example, in benzene and water, a small amount of benzene will be dissolved in the water phase, and vice versa.
Since by definition, immiscible liquids do not interact with each other in any way whatsoever, they will evaporate completely independent of each other.
For separating immiscible liquids like oil and water we use separating funnels.
The principle of separating funnels is that immiscible liquids separate out in layers depending on their densities.
Hence, the correct answer is Option (B) Difference in densities of the two liquids
Additional information:
We can separate a mixture of Kerosene oil and water in the following way.
1- Pour the mixture of kerosene oil and water in a separating funnel
2- Let it stand undisturbed for sometime so that separate layers of oil and water are formed.
3- Open the stopcock of the separating funnel and pour out the lower layer of water carefully.
4- Close the stopcock of the separating funnel as the oil reaches the stop-cock.

Note: This principle of separation of immiscible fluids on the basis of densities also finds application in the extraction of iron from its ore. The lighter kerosene oil is collected at the top of the separating funnel while heavier water will be pore out from the bottom.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




