
What is the special feature of the structure of ethyne?
Answer
504.3k+ views
Hint: “eth” indicates two carbon atoms are present and “yne” indicates that a triple bond is present. Ethyne is a compound containing two carbon atoms connected with a triple bond between them and it is commonly known as Acetylene.
Complete answer:
According to the IUPAC nomenclature, “eth” signifies two carbon compounds and “yne” signifies a triple bond between the two carbon compounds.
Hence, the general formula for alkyne is \[{C_n}{H_{2n}}_{ - 2}\]
Where n is the number of carbon atoms.
The chemical formula for ethyne is \[({C_2}{H_2})\]
The structure is ethyne is as follows:
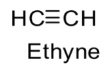
Special feature of the structure of ethyne is the presence of a triple bond.
The structure of ethyne is unstable when it is in its pure form as it is an unsaturated hydrocarbon. The bond angle between the carbon atom i.e. where triple bond is present is almost \[{180^o}\] . Also, the bond length of the \[C - H\] bond is around \[106\] picometers and the bond length of the \[C - C\] bond is around \[120.3\] picometers.
Therefore, in the case of ethyne, every carbon atom is connected with a hydrogen atom by a single bond at one end and with another carbon atom with a triple bond at another end. Ethyne is a symmetrical compound.
Note:
Remember, the presence of a triple bond is a special feature of the structure of ethyne. Some chemical and physical properties of ethyne molecules are that under the standard condition of temperature and pressure (STP) behaves like a colorless gas. It slightly dissolves in water. It does not have any distinct color. Ethyne is a gas and its melting point is \[ - {80.8^o}C\].
Complete answer:
According to the IUPAC nomenclature, “eth” signifies two carbon compounds and “yne” signifies a triple bond between the two carbon compounds.
Hence, the general formula for alkyne is \[{C_n}{H_{2n}}_{ - 2}\]
Where n is the number of carbon atoms.
The chemical formula for ethyne is \[({C_2}{H_2})\]
The structure is ethyne is as follows:

Special feature of the structure of ethyne is the presence of a triple bond.
The structure of ethyne is unstable when it is in its pure form as it is an unsaturated hydrocarbon. The bond angle between the carbon atom i.e. where triple bond is present is almost \[{180^o}\] . Also, the bond length of the \[C - H\] bond is around \[106\] picometers and the bond length of the \[C - C\] bond is around \[120.3\] picometers.
Therefore, in the case of ethyne, every carbon atom is connected with a hydrogen atom by a single bond at one end and with another carbon atom with a triple bond at another end. Ethyne is a symmetrical compound.
Note:
Remember, the presence of a triple bond is a special feature of the structure of ethyne. Some chemical and physical properties of ethyne molecules are that under the standard condition of temperature and pressure (STP) behaves like a colorless gas. It slightly dissolves in water. It does not have any distinct color. Ethyne is a gas and its melting point is \[ - {80.8^o}C\].
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




