
What is the structure of ${K_3}Cr{O_8}$ and what is the oxidation number of Cr here?
Answer
496.8k+ views
Hint: The chemical name of ${K_3}Cr{O_8}$ is Potassium tetraperoxochromate. As the chemical name suggests that there are four peroxy linkages present in the molecule. Peroxy here states that two oxygen bonds are joined by a single bond. In the solution we will see the structure of this compound and the oxidation state of chromium by the substitution method.
Complete answer:
We are given a chemical compound whose chemical formula is ${K_3}Cr{O_8}$ . The chemical name for this compound is Potassium tetraperoxochromate.
The structure of this compound is given as:
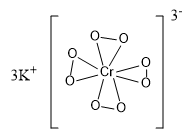
In the above structure we can see that there are four peroxy linkages present in the compound. The structure of the given compound is tetragonal.
Oxidation state of any atom is referred to as the total number of electrons it loses or gains in order to form bonds with other atoms.
Let the oxidation state of Chromium, Cr be $x.$
Oxidation state of Oxygen is $ - 2$ and the charge on complex is $ - 3.$
We will now form an equation to calculate oxidation state of chromium:
$4 \times ( - 2) + x = - 3$
On solving this, we get
$x = + 5$
Therefore, oxidation state of Chromium, Cr in ${K_3}Cr{O_8}$ is $ + 5.$
Note:
D-block elements are also known as transition metals and they form coordination compounds. Coordination compounds are formed when coordinate bonds are formed between the metal atom or ion and ligands. In the above case, the central atom or ion is chromium and the ligands are oxygen. So, oxygen donates its lone pair to the low lying vacant orbitals of the metal.
Complete answer:
We are given a chemical compound whose chemical formula is ${K_3}Cr{O_8}$ . The chemical name for this compound is Potassium tetraperoxochromate.
The structure of this compound is given as:

In the above structure we can see that there are four peroxy linkages present in the compound. The structure of the given compound is tetragonal.
Oxidation state of any atom is referred to as the total number of electrons it loses or gains in order to form bonds with other atoms.
Let the oxidation state of Chromium, Cr be $x.$
Oxidation state of Oxygen is $ - 2$ and the charge on complex is $ - 3.$
We will now form an equation to calculate oxidation state of chromium:
$4 \times ( - 2) + x = - 3$
On solving this, we get
$x = + 5$
Therefore, oxidation state of Chromium, Cr in ${K_3}Cr{O_8}$ is $ + 5.$
Note:
D-block elements are also known as transition metals and they form coordination compounds. Coordination compounds are formed when coordinate bonds are formed between the metal atom or ion and ligands. In the above case, the central atom or ion is chromium and the ligands are oxygen. So, oxygen donates its lone pair to the low lying vacant orbitals of the metal.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




