
Study the structure of maltose and mark the incorrect statement.
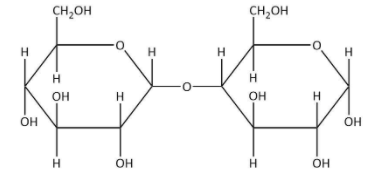
(A) Maltose is composed of two \[\alpha - D - \] glucose units.
(B) \[C - 1\] of one glucose is linked with \[C - 4\] of another unit.
(C) it is a non-reducing sugar.
(D) it is a disaccharide.

Answer
580.8k+ views
Hint: As we know that maltose is a sugar which has free aldehyde group and on hydrolysis it gives two aldohexoses which are monosaccharides. It also has glycosidic linkage.
Maltose has a formula \[{C_{12}}{H_{22}}{O_{11}}\], which is prepared by starch. When starch undergoes hydrolysis in the presence of catalyst amylase, it produces Maltose. When maltose undergoes hydrolysis, it gives two glucose units.
Complete step by step answer:
Now let’s come on the structure of maltose,
As we can see in the above equation Maltose gives two glucose units. The glucose unit consists of six carbon and the \[{1^{st}}\] carbon undergoes cyclization with the \[{5^{th}}\] hydroxyl group. The \[{1^{st}}\] position is also known as anomer because it can arrange itself into two different glucose structures. If the hydroxyl group on\[{1^{st}}\] position is below the plane it is known as \[{\rm{\alpha - D}}\] glucose and if above then it is \[{\rm{\beta - D}}\] glucose. As we can see the below diagram of \[{\rm{\alpha - D}}\] glucose.
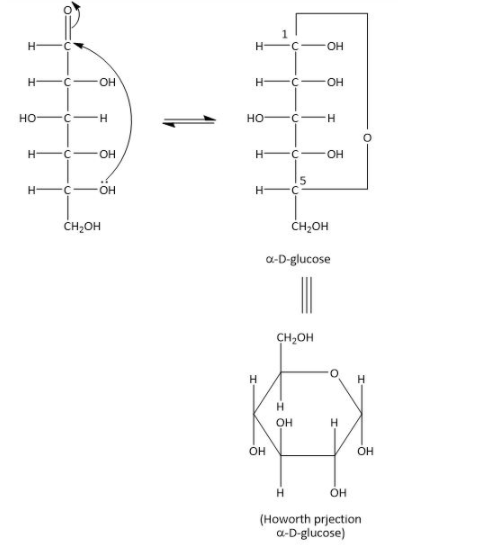
Therefore, the option (A) is correct.
Now come on to the other options we are left with, the two \[{\rm{\alpha - D}}\] glucose units undergo condensation by elimination of water to give maltose. This condensation occurs between \[{1^{st}}\] carbon of one \[{\rm{\alpha - D}}\] glucose and the \[{4^{th}}\] carbon of second \[{\rm{\alpha - D}}\] glucose unit. So, we can say in other words that the condensation occurring between \[C - 1\] of one glucose is linked with \[C - 4\] of another unit also known as \[1 - 4 - \] glycosidic linkage. We can see in the diagram given below-
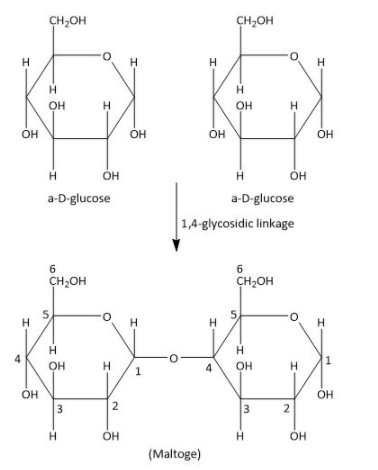
Therefore, the option (B) is correct.
As we can see in the given picture of maltose, \[C - 1\] of one glucose is linked with \[C - 4\] of other units and therefore, one \[\alpha \] position of Second glucose in which \[{4^{th}}\] position linked is free and can be open chain. So maltose is a reducing sugar.
The maltose is formed by the condensation of two glucose units so it is known as disaccharide.
Hence, we can say that the incorrect statement is that maltose is a non-reducing sugar. The correct option is (C).
Note: Mutarotation- when the glucose unit undergoes cyclization it exists in two forms as we can see in the above explanation. These two structures are in equilibrium, this process is known as mutarotation.
Maltose has a formula \[{C_{12}}{H_{22}}{O_{11}}\], which is prepared by starch. When starch undergoes hydrolysis in the presence of catalyst amylase, it produces Maltose. When maltose undergoes hydrolysis, it gives two glucose units.
Complete step by step answer:
Now let’s come on the structure of maltose,
As we can see in the above equation Maltose gives two glucose units. The glucose unit consists of six carbon and the \[{1^{st}}\] carbon undergoes cyclization with the \[{5^{th}}\] hydroxyl group. The \[{1^{st}}\] position is also known as anomer because it can arrange itself into two different glucose structures. If the hydroxyl group on\[{1^{st}}\] position is below the plane it is known as \[{\rm{\alpha - D}}\] glucose and if above then it is \[{\rm{\beta - D}}\] glucose. As we can see the below diagram of \[{\rm{\alpha - D}}\] glucose.

Therefore, the option (A) is correct.
Now come on to the other options we are left with, the two \[{\rm{\alpha - D}}\] glucose units undergo condensation by elimination of water to give maltose. This condensation occurs between \[{1^{st}}\] carbon of one \[{\rm{\alpha - D}}\] glucose and the \[{4^{th}}\] carbon of second \[{\rm{\alpha - D}}\] glucose unit. So, we can say in other words that the condensation occurring between \[C - 1\] of one glucose is linked with \[C - 4\] of another unit also known as \[1 - 4 - \] glycosidic linkage. We can see in the diagram given below-

Therefore, the option (B) is correct.
As we can see in the given picture of maltose, \[C - 1\] of one glucose is linked with \[C - 4\] of other units and therefore, one \[\alpha \] position of Second glucose in which \[{4^{th}}\] position linked is free and can be open chain. So maltose is a reducing sugar.
The maltose is formed by the condensation of two glucose units so it is known as disaccharide.
Hence, we can say that the incorrect statement is that maltose is a non-reducing sugar. The correct option is (C).
Note: Mutarotation- when the glucose unit undergoes cyclization it exists in two forms as we can see in the above explanation. These two structures are in equilibrium, this process is known as mutarotation.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




