
Sucrose molecule is made up of:
A) $\alpha $-D-glucopyranose and $\beta $-D-fructopyranose
B) $\alpha $-D-glucopyranose and $\beta $-D-fructofuranose
C) $\alpha $-D- glucopyranose and $\beta $-D- fructopyranose
D) $\alpha $-D- glucopyranose and $\beta $-D-fructofuranose
Answer
586.2k+ views
Hint: The most abundantly occurring forms of glucose and fructose are pyranose ring form by D-glucose molecule whereas D-fructose occurs in furanose ring form. Sucrose consists of glucose and fructose units in their most common forms by beta glycosidic linkages.
Complete Step by step Solution: Sucrose is a natural polysaccharide with the molecular formula${{\text{C}}_{{\text{12}}}}{{\text{H}}_{{\text{22}}}}{{\text{O}}_{{\text{11}}}}.$ On hydrolysis in acidic medium sucrose gives to two different monosaccharides which are Glucose and fructose.
${{\text{C}}_{{\text{12}}}}{{\text{H}}_{{\text{22}}}}{{\text{O}}_{{\text{11}}}}{\text{ + }}{\kern 1pt} {\kern 1pt} {\kern 1pt} {\kern 1pt} {\kern 1pt} {\kern 1pt} {\kern 1pt} {{\text{H}}_{\text{2}}}{\text{O}} \to {{\text{C}}_{\text{6}}}{{\text{H}}_{{\text{12}}}}{{\text{O}}_{\text{6}}}{\text{ + }}{\kern 1pt} {\kern 1pt} {\kern 1pt} {\kern 1pt} {\kern 1pt} {\kern 1pt} {{\text{C}}_{\text{6}}}{{\text{H}}_{{\text{12}}}}{{\text{O}}_{\text{6}}}$
(Sucrose) (Water) (Glucose) (Fructose)
Predominantly glucose forms pyranose rings as it contains aldehyde (–CHO) group at the end of the chain. Pyranose ring is a six membered ring with five carbon atoms and one oxygen atom in it. So, the glucose exists as a pyranose form in sucrose.
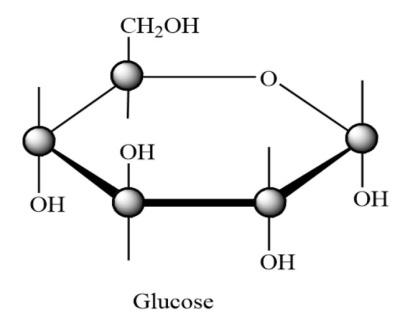
As the hemiacetal group and the hydroxyl (-OH) group lie on opposite sides to each other in glucopyranose ring, this pyranose form of glucose is named as alpha pyranose. Hence the glucose unit in sucrose is
$\alpha $-D-glucopyranose.
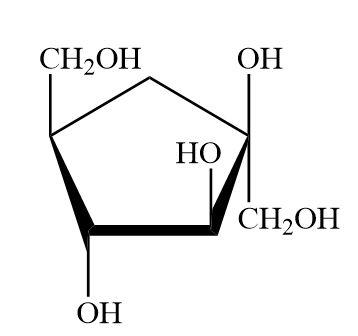
But mostly fructose forms furanose rings as it contains ketone groups at the second carbon of the chain. Furanose ring is a five membered ring with five carbon atoms and one oxygen atom in it. So, the fructose exists as a furanose form in sucrose.
As the hemiacetal group and the hydroxyl (-OH) group lie on the same sides on the glucopyranose ring, this pyranose form of fructose is named as beta furanose. Hence the fructose unit in sucrose is
$\beta $-D-fructofuranose.
The ‘D’ notation is due to the optical rotation value of glucose as glucose. Though glucose and fructose exhibit both L and D forms, the total rotation of both the solutions is ‘D’ (dextrorotatory-clockwise rotation of the incident plane polarized light). The optical rotation value for D-sucrose is +66.37
$^0$.
Additional information: The molecular formula of glucose and fructose is similar which is ${{\text{C}}_{\text{6}}}{{\text{H}}_{{\text{12}}}}{{\text{O}}_{\text{6}}}$ but the structures are different. The difference in molecular structures of glucose and fructose is due to the presence of different carbonyl functional groups on the two molecules. Glucose possesses an aldehyde group on it, whereas fructose possesses a ketone group on it. The Fischer projections of these molecules can be observed to understand the arrangement of carbonyl groups and variations in structures of these compounds though they have the same molecular formula. When this open chain wraps up around itself to form the hemiacetal groups, they form closed ring shaped structures. When a six membered ring forms it is called a pyranose ring and if a five membered ring is formed it is called furanose.
Note: Though glucose exhibits 6 membered pyranose and fructose exhibits 5-membered furanose in sucrose molecules. We should keep in mind that they also occur in other compounds in the form of glucose furanose and fructose pyranose forms.
Complete Step by step Solution: Sucrose is a natural polysaccharide with the molecular formula${{\text{C}}_{{\text{12}}}}{{\text{H}}_{{\text{22}}}}{{\text{O}}_{{\text{11}}}}.$ On hydrolysis in acidic medium sucrose gives to two different monosaccharides which are Glucose and fructose.
${{\text{C}}_{{\text{12}}}}{{\text{H}}_{{\text{22}}}}{{\text{O}}_{{\text{11}}}}{\text{ + }}{\kern 1pt} {\kern 1pt} {\kern 1pt} {\kern 1pt} {\kern 1pt} {\kern 1pt} {\kern 1pt} {{\text{H}}_{\text{2}}}{\text{O}} \to {{\text{C}}_{\text{6}}}{{\text{H}}_{{\text{12}}}}{{\text{O}}_{\text{6}}}{\text{ + }}{\kern 1pt} {\kern 1pt} {\kern 1pt} {\kern 1pt} {\kern 1pt} {\kern 1pt} {{\text{C}}_{\text{6}}}{{\text{H}}_{{\text{12}}}}{{\text{O}}_{\text{6}}}$
(Sucrose) (Water) (Glucose) (Fructose)
Predominantly glucose forms pyranose rings as it contains aldehyde (–CHO) group at the end of the chain. Pyranose ring is a six membered ring with five carbon atoms and one oxygen atom in it. So, the glucose exists as a pyranose form in sucrose.

As the hemiacetal group and the hydroxyl (-OH) group lie on opposite sides to each other in glucopyranose ring, this pyranose form of glucose is named as alpha pyranose. Hence the glucose unit in sucrose is
$\alpha $-D-glucopyranose.
But mostly fructose forms furanose rings as it contains ketone groups at the second carbon of the chain. Furanose ring is a five membered ring with five carbon atoms and one oxygen atom in it. So, the fructose exists as a furanose form in sucrose.
As the hemiacetal group and the hydroxyl (-OH) group lie on the same sides on the glucopyranose ring, this pyranose form of fructose is named as beta furanose. Hence the fructose unit in sucrose is
$\beta $-D-fructofuranose.

The ‘D’ notation is due to the optical rotation value of glucose as glucose. Though glucose and fructose exhibit both L and D forms, the total rotation of both the solutions is ‘D’ (dextrorotatory-clockwise rotation of the incident plane polarized light). The optical rotation value for D-sucrose is +66.37
$^0$.
Additional information: The molecular formula of glucose and fructose is similar which is ${{\text{C}}_{\text{6}}}{{\text{H}}_{{\text{12}}}}{{\text{O}}_{\text{6}}}$ but the structures are different. The difference in molecular structures of glucose and fructose is due to the presence of different carbonyl functional groups on the two molecules. Glucose possesses an aldehyde group on it, whereas fructose possesses a ketone group on it. The Fischer projections of these molecules can be observed to understand the arrangement of carbonyl groups and variations in structures of these compounds though they have the same molecular formula. When this open chain wraps up around itself to form the hemiacetal groups, they form closed ring shaped structures. When a six membered ring forms it is called a pyranose ring and if a five membered ring is formed it is called furanose.
Note: Though glucose exhibits 6 membered pyranose and fructose exhibits 5-membered furanose in sucrose molecules. We should keep in mind that they also occur in other compounds in the form of glucose furanose and fructose pyranose forms.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




