
Terylene is a condensation polymer of …………. and …………….
(A)
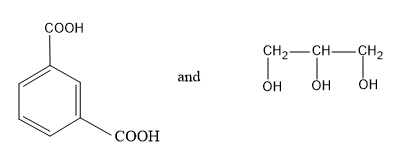
(B)
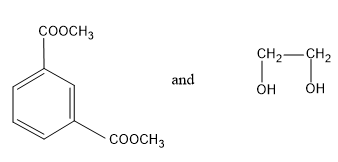
(C)
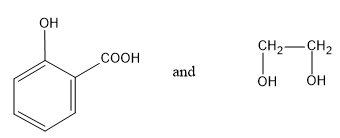
(D) None of these


Answer
564.9k+ views
Hint: Terylene is a polyester and in terylene polymer there is ester linkage in them.
- The monomers of terylene is a dicarboxylic acid and a dioic acid.
Complete step by step answer:
- So in the question we are asked to find the correct monomers which makes the polymer terylene from the given option.
- We are very much familiar with the term polymer from the lower classes and nowadays polymers have become an essential part in our day to day life. We can easily find many processed polymers around us which find application in many fields.
- So before going into the solution of the question, let’s discuss very briefly about, polymers, monomers and condensation polymerization,
- Polymers –polymers are those species which have a very large molecular mass and are formed by either undergoing addition reaction or a condensation polymerization reaction between the monomeric units. Polymers also can be said as the large molecule formed by the repeated addition of a molecule.
- Monomers- these are those units which undergo the polymerization reaction to yield a polymer. These monomeric species add up repeatedly to give the final product. Depending on the monomers involved the polymers are of two types homopolymer and copolymer or heteropolymer.
If the polymerization reaction occurs with the aid of a single monomer it is called as the homopolymer and if two or more monomeric species are involved in the polymerization then they are called as co-polymer.
- Condensation polymerization- this is a type of polymerization in which the monomeric units combine by the elimination of small molecules like water, ammonia, HCl etc. and forms the polymer unit.
Now let’s discuss terylene. Terylene is a condensation co polymer formed by the monomers like ethylene glycol and terephthalic acid. These two monomers undergo condensation reaction and by eliminating a water molecule the polymer chain, terylene is formed. During this polymerization process an ester bond is formed between the monomers.
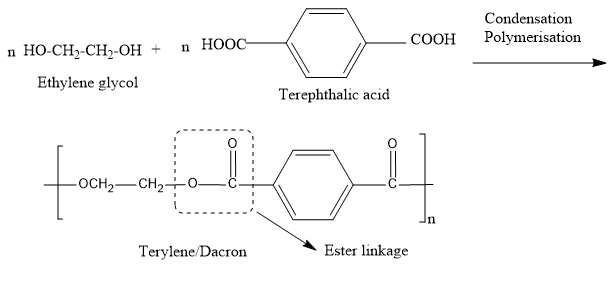
- In this polymerization reaction we use zinc acetate along with antimony trioxide as the catalyst and the polymerization is carried out at a temperature range of 420 - 470 K.
Note: The terylene is also called as Dacron and the terylene fibers have a greater strength since strong intermolecular forces are present.
- The Dacron fibers are blended with the cotton and wool fibers so that the strength of these fibers can be enhanced and terylene is also used as glass reinforcing materials in safety helmets.
- The monomers of terylene is a dicarboxylic acid and a dioic acid.
Complete step by step answer:
- So in the question we are asked to find the correct monomers which makes the polymer terylene from the given option.
- We are very much familiar with the term polymer from the lower classes and nowadays polymers have become an essential part in our day to day life. We can easily find many processed polymers around us which find application in many fields.
- So before going into the solution of the question, let’s discuss very briefly about, polymers, monomers and condensation polymerization,
- Polymers –polymers are those species which have a very large molecular mass and are formed by either undergoing addition reaction or a condensation polymerization reaction between the monomeric units. Polymers also can be said as the large molecule formed by the repeated addition of a molecule.
- Monomers- these are those units which undergo the polymerization reaction to yield a polymer. These monomeric species add up repeatedly to give the final product. Depending on the monomers involved the polymers are of two types homopolymer and copolymer or heteropolymer.
If the polymerization reaction occurs with the aid of a single monomer it is called as the homopolymer and if two or more monomeric species are involved in the polymerization then they are called as co-polymer.
- Condensation polymerization- this is a type of polymerization in which the monomeric units combine by the elimination of small molecules like water, ammonia, HCl etc. and forms the polymer unit.
Now let’s discuss terylene. Terylene is a condensation co polymer formed by the monomers like ethylene glycol and terephthalic acid. These two monomers undergo condensation reaction and by eliminating a water molecule the polymer chain, terylene is formed. During this polymerization process an ester bond is formed between the monomers.

- In this polymerization reaction we use zinc acetate along with antimony trioxide as the catalyst and the polymerization is carried out at a temperature range of 420 - 470 K.
Note: The terylene is also called as Dacron and the terylene fibers have a greater strength since strong intermolecular forces are present.
- The Dacron fibers are blended with the cotton and wool fibers so that the strength of these fibers can be enhanced and terylene is also used as glass reinforcing materials in safety helmets.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




