
The acidic strength order is:
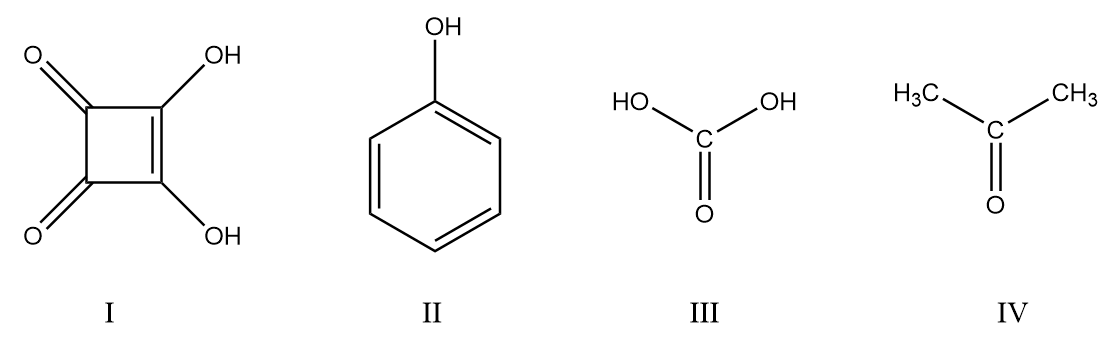
A. $I > IV > II > III$
B. $III > I > II > IV$
C. $II > III > II > IV$
D. $I > III > II > IV$

Answer
511.8k+ views
Hint :According to the Bronsted Lowry concept, an acid is a substance which donates protons to form its conjugate base whereas a base is a substance which accepts a proton to form its conjugate acid. The corresponding conjugate base of strong Bronsted acid is weak whereas the Bronsted acids which are weak consist of strong conjugate bases.
Complete Step By Step Answer:
As per Bronsted Lowry concept, with increasing acidity, the basicity of conjugate bases decreases and their stability increases. Therefore, a more stable conjugate base will have higher acidic strength. Hence, acidic strength among given molecules can be compared on the basis of stability of their respective conjugate base as follows:
Compound-I:
The conjugate base of the given compound is as follows:
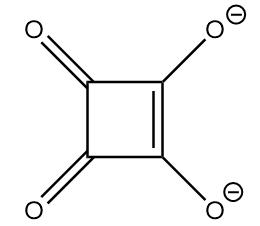
As the compound consists of dianion with a conjugated double bond system, it exists in four equivalent resonating structures. The resonating structures are as follows:
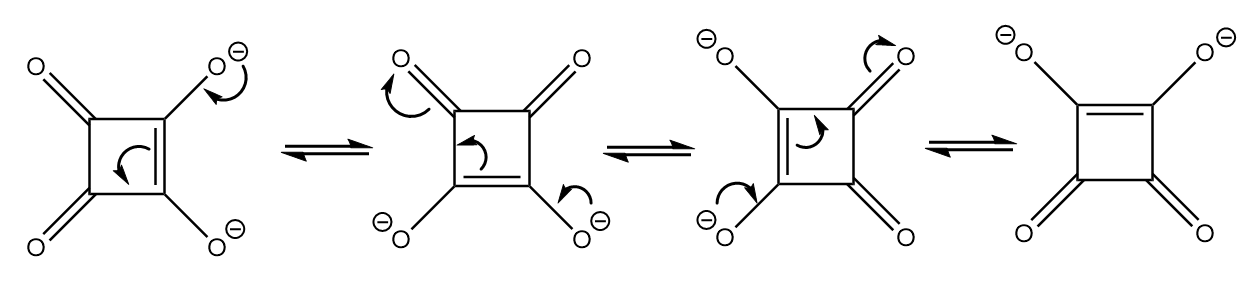
Therefore, it is the most stable conjugate base.
Compound-II:
The conjugate base of the given compound is as follows:
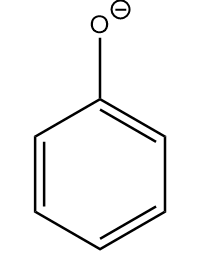
As the compound consists of an aromatic ring with a conjugated double bond system, so it is stabilized due to resonance. The resonating structures are as follows:
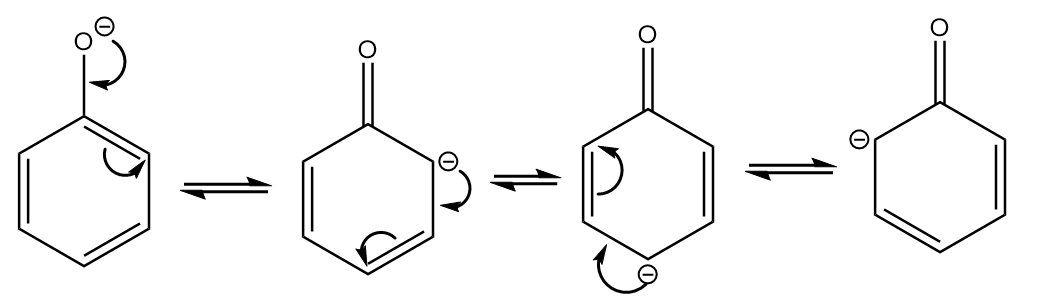
Although it forms more resonating structures than compound-III, it is less stable because the negative charge resides on less electronegative carbon atoms. Therefore, it is placed at third position in the stability order.
Compound-III:
The conjugate base of the compound is as follows:
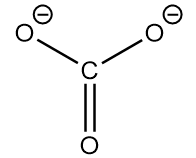
As the ring consists of dianion and conjugated pi electron system. So, it exists in its three equivalent resonating structures which are as follows:
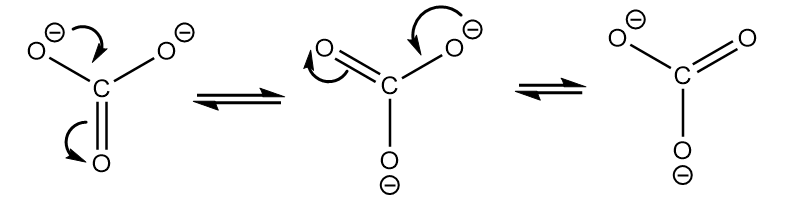
As the negative charge resides on more electronegative oxygen atoms, it is more stable than phenoxide ions formed in compound-II but less stable than the conjugate base of compound-I due to less resonating structures.
Compound-IV:
The conjugate base of the compound is as follows:
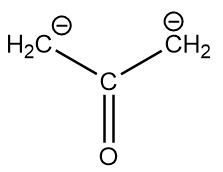
The resonating structures of the given conjugate base are as follows:
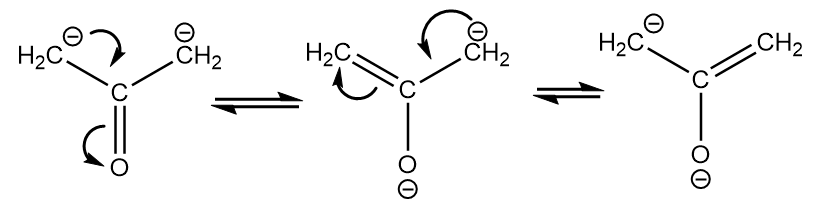
It is the least stable conjugate base because of a lesser number of resonating structures and the negative charge resides on the less electronegative carbon atoms.
Hence, the order of stability of conjugate bases formed from the given compound is as follows:
$I > III > II > IV$
Therefore, the order of acidic strength is $I > III > II > IV$. So, option (D) is the correct answer.
Note :
Remember that a pair of substance or molecules which differ only by the presence of a proton i.e., ${H^ + }$ ion is known as a conjugate acid-base pair. In simple words, the molecules which can be formed from each other by gaining or losing a proton are termed as conjugate acid-base pairs. This concept has wide application to compare relative strengths of acids and bases.
Complete Step By Step Answer:
As per Bronsted Lowry concept, with increasing acidity, the basicity of conjugate bases decreases and their stability increases. Therefore, a more stable conjugate base will have higher acidic strength. Hence, acidic strength among given molecules can be compared on the basis of stability of their respective conjugate base as follows:
Compound-I:
The conjugate base of the given compound is as follows:

As the compound consists of dianion with a conjugated double bond system, it exists in four equivalent resonating structures. The resonating structures are as follows:

Therefore, it is the most stable conjugate base.
Compound-II:
The conjugate base of the given compound is as follows:

As the compound consists of an aromatic ring with a conjugated double bond system, so it is stabilized due to resonance. The resonating structures are as follows:

Although it forms more resonating structures than compound-III, it is less stable because the negative charge resides on less electronegative carbon atoms. Therefore, it is placed at third position in the stability order.
Compound-III:
The conjugate base of the compound is as follows:

As the ring consists of dianion and conjugated pi electron system. So, it exists in its three equivalent resonating structures which are as follows:

As the negative charge resides on more electronegative oxygen atoms, it is more stable than phenoxide ions formed in compound-II but less stable than the conjugate base of compound-I due to less resonating structures.
Compound-IV:
The conjugate base of the compound is as follows:

The resonating structures of the given conjugate base are as follows:

It is the least stable conjugate base because of a lesser number of resonating structures and the negative charge resides on the less electronegative carbon atoms.
Hence, the order of stability of conjugate bases formed from the given compound is as follows:
$I > III > II > IV$
Therefore, the order of acidic strength is $I > III > II > IV$. So, option (D) is the correct answer.
Note :
Remember that a pair of substance or molecules which differ only by the presence of a proton i.e., ${H^ + }$ ion is known as a conjugate acid-base pair. In simple words, the molecules which can be formed from each other by gaining or losing a proton are termed as conjugate acid-base pairs. This concept has wide application to compare relative strengths of acids and bases.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




