
The alkene formed as a major product in the above elimination reactions is:
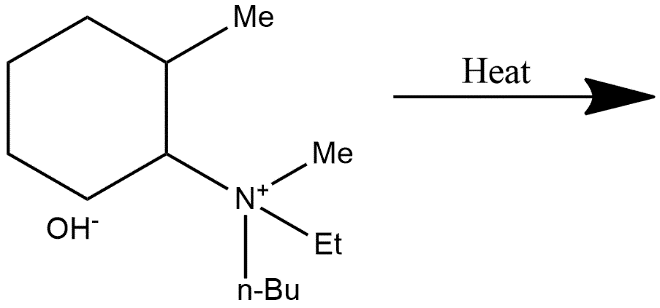
A.
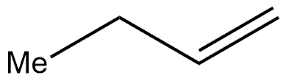
B.

C.
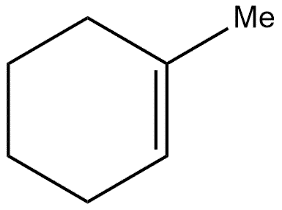
D.





Answer
531.6k+ views
Hint: The given reaction can be explained on the basis of Hofmann elimination which can be defined as the process of creating tertiary amines and alkenes i.e. amines and alkenes having three groups attached with itself from the treatment of quaternary ammonium with excess methyl iodide.
Complete answer:
This process can also be referred to as exhaustive methylation. The Hofmann elimination process is named after the scientist who discovered this process and this is discovered by German chemist August Wilhelm Von Hofmann.
The Hofmann rule states that when there is an asymmetrical amine present in the molecule then the major product of that alkene is least substituted in nature and we can also say that as a result it also behaves as a least stable product. The given compound is quaternary ammonium salt in which four $\beta $ hydrogen atoms and on further heating these quaternary ammonium salts it gives Hofmann elimination reactions in which it abstract most acidic hydrogen and major product formed is least substituted alkene i.e.
$C{{H}_{2}}=C{{H}_{2}}C{{H}_{2}}=C{{H}_{2}}$, from this we can easily conclude that option A is correct.
Note:
If there are no beta hydrogen atoms present in the molecule then Hoffmann elimination reaction is not possible to occur. The most important example of the Hofmann elimination process or the exhaustive methylation process is the synthesis of trans-cyclooctene.
Complete answer:
This process can also be referred to as exhaustive methylation. The Hofmann elimination process is named after the scientist who discovered this process and this is discovered by German chemist August Wilhelm Von Hofmann.
The Hofmann rule states that when there is an asymmetrical amine present in the molecule then the major product of that alkene is least substituted in nature and we can also say that as a result it also behaves as a least stable product. The given compound is quaternary ammonium salt in which four $\beta $ hydrogen atoms and on further heating these quaternary ammonium salts it gives Hofmann elimination reactions in which it abstract most acidic hydrogen and major product formed is least substituted alkene i.e.
$C{{H}_{2}}=C{{H}_{2}}C{{H}_{2}}=C{{H}_{2}}$, from this we can easily conclude that option A is correct.
Note:
If there are no beta hydrogen atoms present in the molecule then Hoffmann elimination reaction is not possible to occur. The most important example of the Hofmann elimination process or the exhaustive methylation process is the synthesis of trans-cyclooctene.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




