
The alkyl radical with most stability is:
A. Ethyl radical
B. Methyl radical
C. \[{2^ \circ }\] – butyl radical
D. \[{3^ \circ }\] – butyl radical
Answer
582k+ views
Hint: To answer this question you need to know the molecular formula and structure of all the radicals. Alkyls are basically alkane with one hydrogen missing. So, if we know alkanes then we can know alkyls of ethyl and methyl.
Complete step by step answer:
In organic chemistry, an alkyl substituent is basically alkane with one hydrogen missing. For example, cycloalkyl is derived from cycloalkane with one hydrogen atom removed and its general formula is \[{C_n}{H_{2n - 1}}\] . The acyclic alkyl has the general formula of \[{C_n}{H_{2n + 1}}\] . The smallest alkyl group is methyl having formula \[C{H_3}\] .
Alkyl radical is any series of universal groups of the general formula \[{C_n}{H_{2n + 1}}\] derived from aliphatic hydrocarbons. In order to understand radical stability we must first understand the structure of alkyl radicals. They have a trigonal planar geometry associated with \[s{p^2}\] hybridisation.
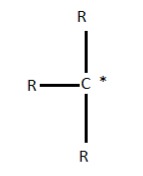
Ethyl radical has a molecular formula \[{C_2}{H_5}\] and it is derived from ethane ( \[{C_2}{H_6}\] ). It is abbreviated as Et. The prefix “eth-” is used to indicate the presence of two carbon atoms in the molecule. The structure of ethyl radical is as given below.
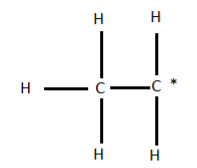
Methyl Radical is an organic compound with the chemical formula \[C{H_3}^*\] . It is a metastable colourless gas and acts as either a strong oxidant or a strong reductant and is quite corrosive to metals. The structure of methyl radical is as follows:
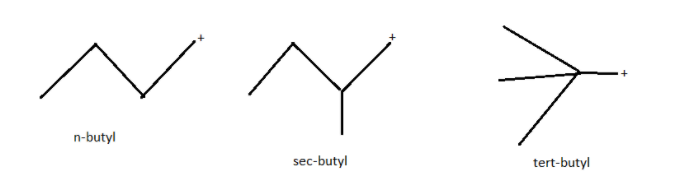
Butyl is a four carbon alkyl radical or substituent group with general chemical formula as \[{C_4}{H_9}\] derived from either of the two isomers of butane. If any atom connects at one of the two terminal carbon atoms then it is normal butyl or n-butyl. If it connects at one of the non terminal carbon atoms it is a secondary butyl or sec-butyl having molecular formula \[C{H_3} - C{H_2} - CH(C{H_3}) - \] . If it connects at the central carbon then it is tertiary butyl, tert-butyl or t-butyl with molecular formula \[{(C{H_3})_3}C - \] . The structures of all types of butyls are as given below.
The general trend in radical stability is \[{3^ \circ }\] > \[{2^ \circ }\] > \[{1^ \circ }\] >methyl. Therefore, the highest stability is in \[{3^ \circ }\] butyl radical. This is due to no bond resonance of adjacent hydrogens with the positive charge. Tertiary free radicals like \[{3^ \circ }\] butyl have 3 methyl groups and 9 hyper conjugative hydrogens which makes it most stable.
Therefore, the answer is option D.
Note:
Having knowledge of alkanes can help here with alkyls. The 2 degree and 3 degree butyl properties should be known and the difference between them so that there is no confusion on the number of carbon and hydrogen atoms present in the molecules.
Complete step by step answer:
In organic chemistry, an alkyl substituent is basically alkane with one hydrogen missing. For example, cycloalkyl is derived from cycloalkane with one hydrogen atom removed and its general formula is \[{C_n}{H_{2n - 1}}\] . The acyclic alkyl has the general formula of \[{C_n}{H_{2n + 1}}\] . The smallest alkyl group is methyl having formula \[C{H_3}\] .
Alkyl radical is any series of universal groups of the general formula \[{C_n}{H_{2n + 1}}\] derived from aliphatic hydrocarbons. In order to understand radical stability we must first understand the structure of alkyl radicals. They have a trigonal planar geometry associated with \[s{p^2}\] hybridisation.

Ethyl radical has a molecular formula \[{C_2}{H_5}\] and it is derived from ethane ( \[{C_2}{H_6}\] ). It is abbreviated as Et. The prefix “eth-” is used to indicate the presence of two carbon atoms in the molecule. The structure of ethyl radical is as given below.

Methyl Radical is an organic compound with the chemical formula \[C{H_3}^*\] . It is a metastable colourless gas and acts as either a strong oxidant or a strong reductant and is quite corrosive to metals. The structure of methyl radical is as follows:

Butyl is a four carbon alkyl radical or substituent group with general chemical formula as \[{C_4}{H_9}\] derived from either of the two isomers of butane. If any atom connects at one of the two terminal carbon atoms then it is normal butyl or n-butyl. If it connects at one of the non terminal carbon atoms it is a secondary butyl or sec-butyl having molecular formula \[C{H_3} - C{H_2} - CH(C{H_3}) - \] . If it connects at the central carbon then it is tertiary butyl, tert-butyl or t-butyl with molecular formula \[{(C{H_3})_3}C - \] . The structures of all types of butyls are as given below.

The general trend in radical stability is \[{3^ \circ }\] > \[{2^ \circ }\] > \[{1^ \circ }\] >methyl. Therefore, the highest stability is in \[{3^ \circ }\] butyl radical. This is due to no bond resonance of adjacent hydrogens with the positive charge. Tertiary free radicals like \[{3^ \circ }\] butyl have 3 methyl groups and 9 hyper conjugative hydrogens which makes it most stable.
Therefore, the answer is option D.
Note:
Having knowledge of alkanes can help here with alkyls. The 2 degree and 3 degree butyl properties should be known and the difference between them so that there is no confusion on the number of carbon and hydrogen atoms present in the molecules.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




