
The basicity of
(I) o—toluidine
(II) m—toluidine
(III) p—toluidine
(IV) aniline
follow the order:
A. I>II>III>IV
B. IV>III>II>I
C. III>II>IV>I
D. I>IV>II>III
Answer
233.1k+ views
Hint: According to the concept of Lewis acid-base theory the compound or molecule can accept hydrogen ion is known as base. Higher the tendency to accept hydrogen ion higher will be the basicity of that compound.
Complete step by step solution:
Toluidine is an aromatic amine. The basicity of the aromatic amine is depending upon the availability of the lone pair. Higher the lone pair availability higher the donating ability of the lone pair as well as accept tendency of hydrogen ions.
In case of aromatic amine, the lone pair undergoes conjugation with the benzene . As a result, lone pair availability decreases. Therefore, in presence of an electron donating group increases the tendency of lone pair availability as well as basicity.
The structures of o—toluidine, m—toluidine, p—toluidine, aniline as follows,
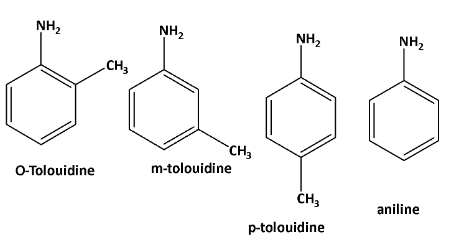
Now \[{\text{ - C}}{{\text{H}}_{\text{3}}}\] is an electron donating group. due to the positive inductive effect of the methyl group the lone pair of amine availability increases as well as the basicity.
In the case of o—toluidine, due to the steric hindrance of the methyl group , after accepting the hydrogen ion the ammonium ion gets out of the plane. As a result, corresponding conjugate acid stability gets decreased. That is why the basicity of o—toluidine is least among others.
In the case of p—toluidine due the hyperconjugation of methyl groups the basicity increases greater than others.
So, the overall order is, p—toluidine> m—toluidine> aniline> o—toluidine,
So, the correct option is, C. III>II>IV>I
Note: Acidity of an organic compound is depending upon the electron deficiency of the hydrogen atom. Higher the electron deficiency of the hydrogen higher will be the acidity character of that hydrogen.
Now to be an acidic hydrogen that hydrogen should be attached with a high electronegative group or electron withdrawing group. Higher the electronegativity of the group, higher will be the electron deficiency of the hydrogen, attached with that group as well as the acidity.
Complete step by step solution:
Toluidine is an aromatic amine. The basicity of the aromatic amine is depending upon the availability of the lone pair. Higher the lone pair availability higher the donating ability of the lone pair as well as accept tendency of hydrogen ions.
In case of aromatic amine, the lone pair undergoes conjugation with the benzene . As a result, lone pair availability decreases. Therefore, in presence of an electron donating group increases the tendency of lone pair availability as well as basicity.
The structures of o—toluidine, m—toluidine, p—toluidine, aniline as follows,

Now \[{\text{ - C}}{{\text{H}}_{\text{3}}}\] is an electron donating group. due to the positive inductive effect of the methyl group the lone pair of amine availability increases as well as the basicity.
In the case of o—toluidine, due to the steric hindrance of the methyl group , after accepting the hydrogen ion the ammonium ion gets out of the plane. As a result, corresponding conjugate acid stability gets decreased. That is why the basicity of o—toluidine is least among others.
In the case of p—toluidine due the hyperconjugation of methyl groups the basicity increases greater than others.
So, the overall order is, p—toluidine> m—toluidine> aniline> o—toluidine,
So, the correct option is, C. III>II>IV>I
Note: Acidity of an organic compound is depending upon the electron deficiency of the hydrogen atom. Higher the electron deficiency of the hydrogen higher will be the acidity character of that hydrogen.
Now to be an acidic hydrogen that hydrogen should be attached with a high electronegative group or electron withdrawing group. Higher the electronegativity of the group, higher will be the electron deficiency of the hydrogen, attached with that group as well as the acidity.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




