
The bond order of \[Cl-O\] bond in \[ClO_{4}^{-}\] ion and the effective charge on each oxygen atom respectively are:
A. 1.75,-1
B. 1.75, -0.25
C. 1.5, -0.5
D. 2.0, -0.5
Answer
522k+ views
Hint: To solve these types of questions we should first draw the Lewis structure. And then after it, we then count the number of total bonds and then divide it by the number of elements in which these bonds are made with the central atom.
Step by step answer:
We can solve this question, by first drawing the Lewis dot structure of \[ClO_{4}^{-}\] ion.
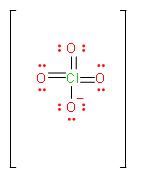
In the above figure, the total number of bonds is 7. And if we try to draw different structures, these bonds will rearrange themselves between four elements. Or we can calculate it by counting the number of resonance structures this molecule can make.
$Bond\,order=\dfrac{total\,number\,of\,bonds}{total\,number\,of\,resonance\,structures}$\[\begin{align}
& Formal\text{ }charge\,of\,first\,oxygen=6-6-\dfrac{2}{2}=-1 \\
& Formal\text{ }charge\,of\,\sec ond,\,third\,and\,fourth\,oxygen=6-4-\dfrac{4}{2}=0 \\
\end{align}\]
\[Bond\,order=\dfrac{7}{4}=1.75\]
So, from the above calculation we found that the bond order of perchlorate ion is 1.75.
Now, we will calculate formal charge on each oxygen atom. First we assign numbers to oxygen atoms.
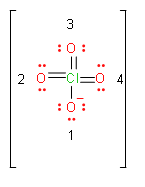
\[Formal\text{ }charge=\text{ }valence\text{ }electrons-unbonded\,electrons-\dfrac{1}{2}bonded\,electrons\]
\[\begin{align}
& Formal\text{ }charge\,of\,first\,oxygen=6-6-\dfrac{2}{2}=-1 \\
& Formal\text{ }charge\,of\,\sec ond,\,third\,and\,fourth\,oxygen=6-4-\dfrac{4}{2}=0 \\
\end{align}\]
So, effective charge on oxygen will become -0.25.
From the above discussion and calculation, we can now say that the answer of this question is option B.
Additional information:
We should know that perchlorate is a chemical compound containing the perchlorate ion \[ClO_{4}^{-}\]. The majority of perchlorates are commercially produced salts. They are mainly used for propellants, exploiting properties as powerful oxidizing agents and to control static electricity in food packaging. Most perchlorates are colourless solids that are soluble in water.
Note: We should also know about bond length. It is defined as the distance between the centres of two covalently bonded atoms. The length of the bond is determined by the number of bonded electrons (the bond order). If bond order is high, there will be stronger pull between two atoms and there will be shorter bond length. Therefore, bond length increases in the following order: triple bond < double bond < single bond.
Step by step answer:
We can solve this question, by first drawing the Lewis dot structure of \[ClO_{4}^{-}\] ion.

In the above figure, the total number of bonds is 7. And if we try to draw different structures, these bonds will rearrange themselves between four elements. Or we can calculate it by counting the number of resonance structures this molecule can make.
$Bond\,order=\dfrac{total\,number\,of\,bonds}{total\,number\,of\,resonance\,structures}$\[\begin{align}
& Formal\text{ }charge\,of\,first\,oxygen=6-6-\dfrac{2}{2}=-1 \\
& Formal\text{ }charge\,of\,\sec ond,\,third\,and\,fourth\,oxygen=6-4-\dfrac{4}{2}=0 \\
\end{align}\]
\[Bond\,order=\dfrac{7}{4}=1.75\]
So, from the above calculation we found that the bond order of perchlorate ion is 1.75.
Now, we will calculate formal charge on each oxygen atom. First we assign numbers to oxygen atoms.

\[Formal\text{ }charge=\text{ }valence\text{ }electrons-unbonded\,electrons-\dfrac{1}{2}bonded\,electrons\]
\[\begin{align}
& Formal\text{ }charge\,of\,first\,oxygen=6-6-\dfrac{2}{2}=-1 \\
& Formal\text{ }charge\,of\,\sec ond,\,third\,and\,fourth\,oxygen=6-4-\dfrac{4}{2}=0 \\
\end{align}\]
So, effective charge on oxygen will become -0.25.
From the above discussion and calculation, we can now say that the answer of this question is option B.
Additional information:
We should know that perchlorate is a chemical compound containing the perchlorate ion \[ClO_{4}^{-}\]. The majority of perchlorates are commercially produced salts. They are mainly used for propellants, exploiting properties as powerful oxidizing agents and to control static electricity in food packaging. Most perchlorates are colourless solids that are soluble in water.
Note: We should also know about bond length. It is defined as the distance between the centres of two covalently bonded atoms. The length of the bond is determined by the number of bonded electrons (the bond order). If bond order is high, there will be stronger pull between two atoms and there will be shorter bond length. Therefore, bond length increases in the following order: triple bond < double bond < single bond.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




