
The catalyst used in the Gattermann Koch reaction is:
(a) anhydrous $AlC{{l}_{3}}$ and $C{{u}_{2}}C{{l}_{2}}$
(b) anhydrous $AlC{{l}_{3}}$
(c) $C{{u}_{2}}C{{l}_{2}}$
(d) $CuC{{l}_{2}}$
Answer
534.3k+ views
Hint: The Gattermann Koch reaction is that reaction in which the ring compounds undergo formylation when treated with CO and HCl in the presence of two catalysts. Both are chlorides, the element with one chloride belongs to the boron family and the element with the other chloride is a transition element and with the help of this you can easily identify the catalyst. Now answer the statement accordingly.
Complete step by step solution: First of all, let’s discuss what is a catalyst. A catalyst is a substance which increases the rate of the chemical reaction and lowers the activation energy. They do not undergo any chemical change in its own composition i.e. it undergoes temporary changes and is not permanent.
Now discuss what is Gattermann-Koch reaction. In the Gattermann-Koch reaction, aromatic compounds are treated with the carbon monoxide and hydrochloric acid in the presence of a Lewis acid catalyst i.e. anhydrous aluminum chloride and $C{{u}_{2}}C{{l}_{2}}$ and finally, results in the formation of an aldehyde. The chemical reaction is supposed to occurs as follows;
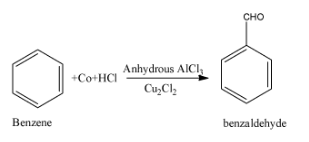
Thus, the catalyst used in the Gattermann Koch reaction is anhydrous $AlC{{l}_{3}}$ and $C{{u}_{2}}C{{l}_{2}}$.
So, option (a) is correct.
Note: Catalyst is not consumed during the reaction but is regenerated at the end of reaction. It is so because the catalyst is used only in one step and is regenerated in the other steps. Thus, remains unused in the reaction and doesn’t undergo any chemical and permanent changes during the reaction.
Complete step by step solution: First of all, let’s discuss what is a catalyst. A catalyst is a substance which increases the rate of the chemical reaction and lowers the activation energy. They do not undergo any chemical change in its own composition i.e. it undergoes temporary changes and is not permanent.
Now discuss what is Gattermann-Koch reaction. In the Gattermann-Koch reaction, aromatic compounds are treated with the carbon monoxide and hydrochloric acid in the presence of a Lewis acid catalyst i.e. anhydrous aluminum chloride and $C{{u}_{2}}C{{l}_{2}}$ and finally, results in the formation of an aldehyde. The chemical reaction is supposed to occurs as follows;

Thus, the catalyst used in the Gattermann Koch reaction is anhydrous $AlC{{l}_{3}}$ and $C{{u}_{2}}C{{l}_{2}}$.
So, option (a) is correct.
Note: Catalyst is not consumed during the reaction but is regenerated at the end of reaction. It is so because the catalyst is used only in one step and is regenerated in the other steps. Thus, remains unused in the reaction and doesn’t undergo any chemical and permanent changes during the reaction.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




