
The $C{{O}_{2}}$ content by volume, in the atmospheric air, is about
(a)3.34%
(b)4%
(c)0.0314%
(d)2.1%
Answer
584.1k+ views
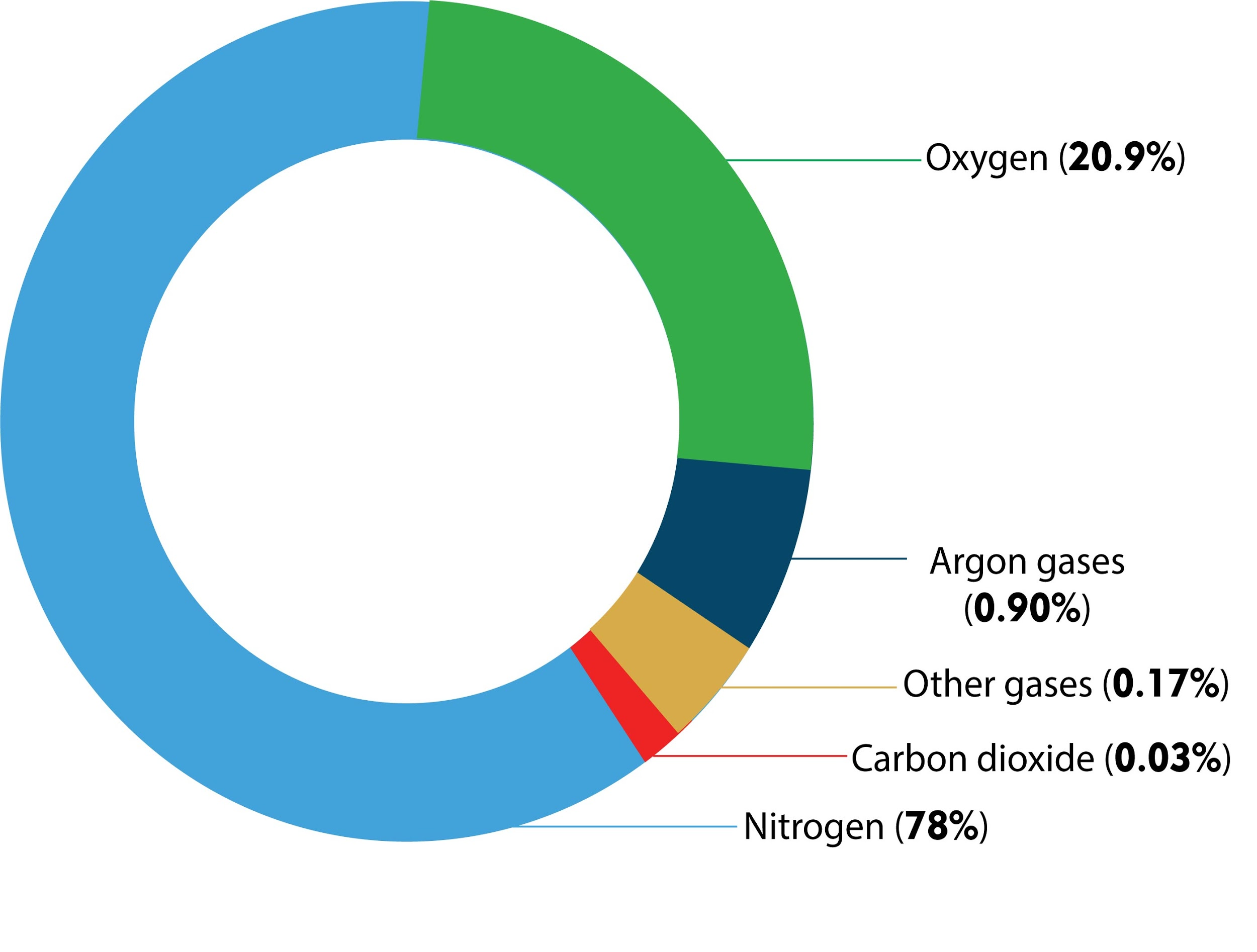
Hint: In the atmosphere, the composition of nitrogen is 78.08%, oxygen 20.95%, argon 0.93%, and rest for the carbon dioxide. Carbon dioxide is essential for a green plant for photosynthesis.
Complete answer:
The atmosphere is a mixture of permanent and variable gases and aerosols. Where permanent constituents gases like nitrogen, oxygen, argon, neon, helium, krypton, hydrogen should remain essentially constant by percent. Variable constituents are those gases that have to change concentration over a finite time, for example, water vapor, carbon dioxide, methane, ozone, etc.
Additional Information: -Carbon dioxide is a colorless and odorless gas where the carbon atoms are covalently double bonded to two oxygen atoms.
-Carbon dioxide is generated as a by-product of the combustion of fossil fuel or the burning of vegetable matter, among other chemical processes.
-Carbon dioxide has no liquid state at a pressure below 5.1 atm but at 1 atm the gas deposits directly to a solid start temperature below -78˚C and known as dry ice.
-Carbon dioxide is a waste product in humans where 1% of toxic carbon dioxide causes drowsiness and 7% to 10% of toxicity causes dizziness, headache, visual and hearing dysfunction, and unconsciousness within a few minutes.
So, the correct answer is, ‘0.0314%.’
Note: -Carbon dioxide is also used as a food additive used as a propellant and acidity regulator in the food industry.
-Carbon dioxide can be mixed with argon as a shielding gas used to prevent atmospheric contamination of molten metal in electric arc welding processes.
-The carbon dioxide used in extinguishing flames especially for electrical fires contains liquid carbon dioxide under pressure.
Complete answer:
The atmosphere is a mixture of permanent and variable gases and aerosols. Where permanent constituents gases like nitrogen, oxygen, argon, neon, helium, krypton, hydrogen should remain essentially constant by percent. Variable constituents are those gases that have to change concentration over a finite time, for example, water vapor, carbon dioxide, methane, ozone, etc.
Additional Information: -Carbon dioxide is a colorless and odorless gas where the carbon atoms are covalently double bonded to two oxygen atoms.
-Carbon dioxide is generated as a by-product of the combustion of fossil fuel or the burning of vegetable matter, among other chemical processes.
-Carbon dioxide has no liquid state at a pressure below 5.1 atm but at 1 atm the gas deposits directly to a solid start temperature below -78˚C and known as dry ice.
-Carbon dioxide is a waste product in humans where 1% of toxic carbon dioxide causes drowsiness and 7% to 10% of toxicity causes dizziness, headache, visual and hearing dysfunction, and unconsciousness within a few minutes.
So, the correct answer is, ‘0.0314%.’
Note: -Carbon dioxide is also used as a food additive used as a propellant and acidity regulator in the food industry.
-Carbon dioxide can be mixed with argon as a shielding gas used to prevent atmospheric contamination of molten metal in electric arc welding processes.
-The carbon dioxide used in extinguishing flames especially for electrical fires contains liquid carbon dioxide under pressure.

Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

A moving boat is observed from the top of a 150 m high class 10 maths CBSE




