
The compound ‘X’ gives brisk effervescence of \[C{O_2}\] on treatment with \[NaHC{O_3}\] and when treated with \[LiAl{H_4}\] gives ethyl alcohol. Therefore ‘X’ may be..
(A) \[C{H_3}COC{H_3}\]
(B) \[C{H_3}C{H_2}CHO\]
(C) \[C{H_3}CHO\]
(D) \[C{H_3}COOH\]
Answer
594k+ views
Hint:
Lithium aluminum hydride reacts with carbonyl carbon to give hydroxyl group in product. Sodium bicarbonate is a base and it reacts with acids to give salts. Both aldehydes and carboxylic acid groups will get reduced by lithium aluminum hydride.
Complete step by step answer:
It is proved from experiments that organic acids produce carbon dioxide gas when they react with sodium bicarbonate solution. So, salt of acid and carbon dioxide gas are produced as products. The release of carbon dioxide gas gives brisk effervescence. Let’s see which of the given compounds will give brisk effervescence when treated with sodium bicarbonate.
- \[C{H_3}COC{H_3}\] contains only ketone functional group, \[C{H_3}C{H_2}CHO\] and \[C{H_3}CHO\] contains aldehyde functional group. So, these three compounds will not react with sodium bicarbonate.
- \[C{H_3}COOH\] has a carboxylic acid group and is an organic acid, so it will react with sodium bicarbonate to produce brisk effervescence of carbon dioxide gas. The reaction is given as below.
\[\mathop {C{H_3}COOH}\limits_{{\text{Acetic acid}}} + NaHC{O_3} \to \mathop {C{H_3}COONa}\limits_{Sodium{\text{ Acetate}}} + C{O_2}\]
Now, Lithium aluminum hydride is a reducing agent and will reduce any carbonyl carbon to give hydroxyl group on it.
Let’s see how the given compounds will react with Lithium aluminum hydride.
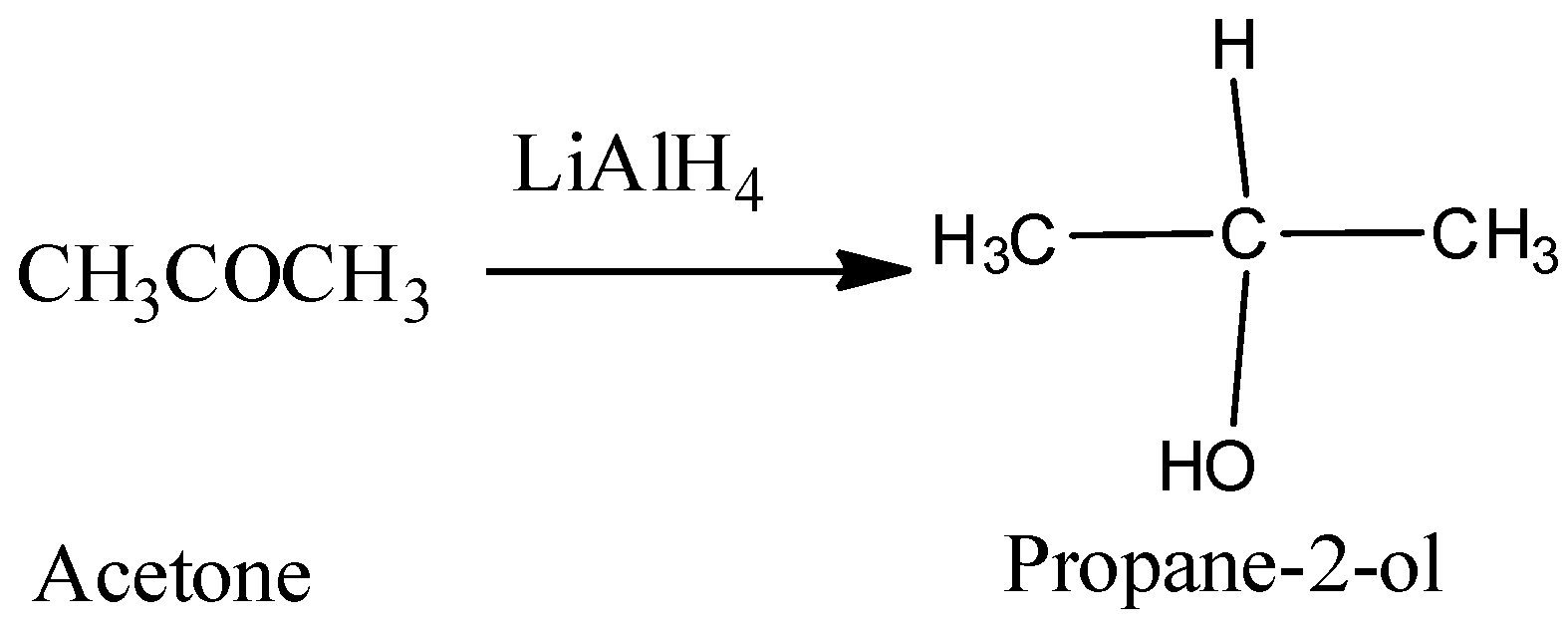
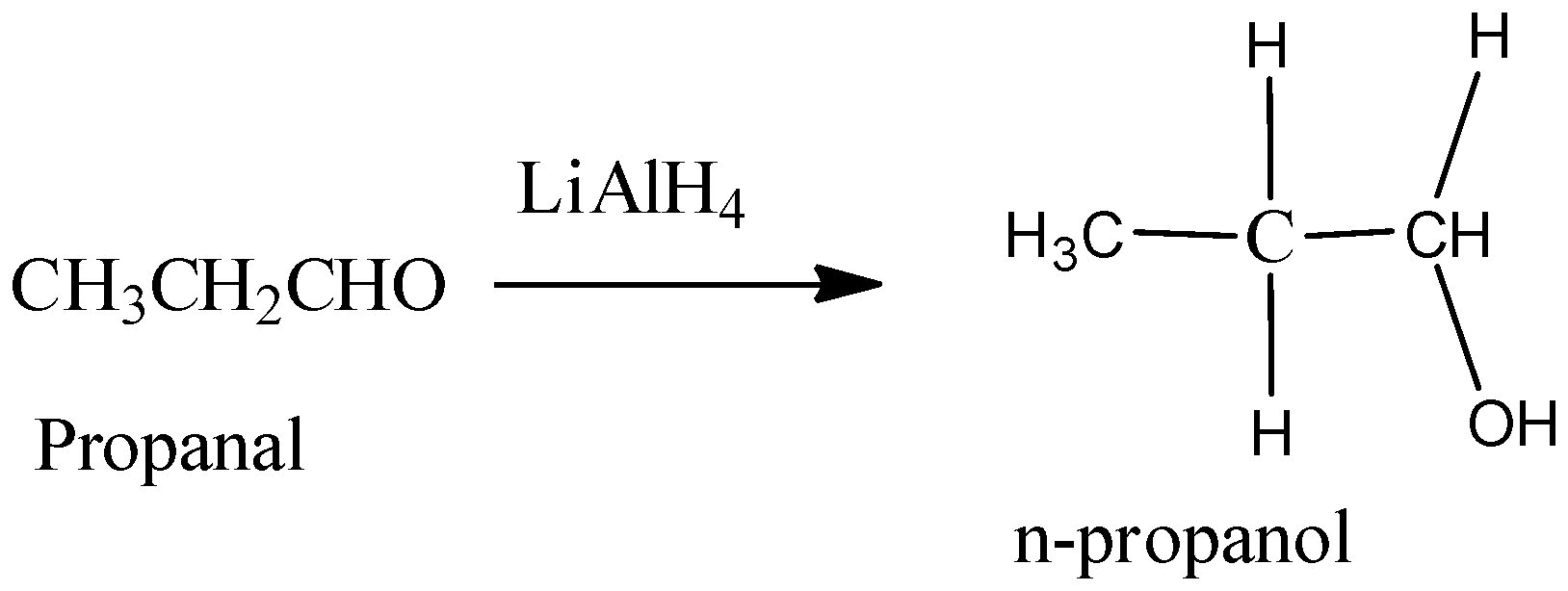
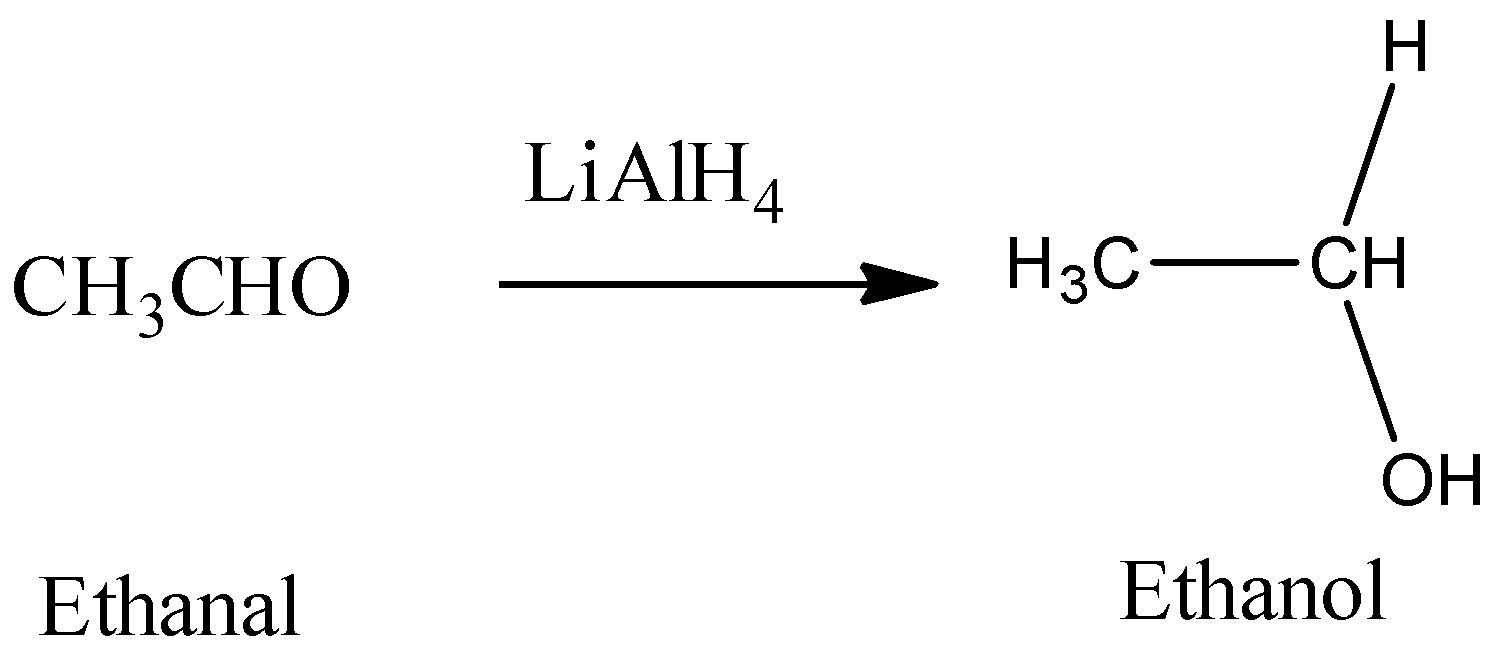
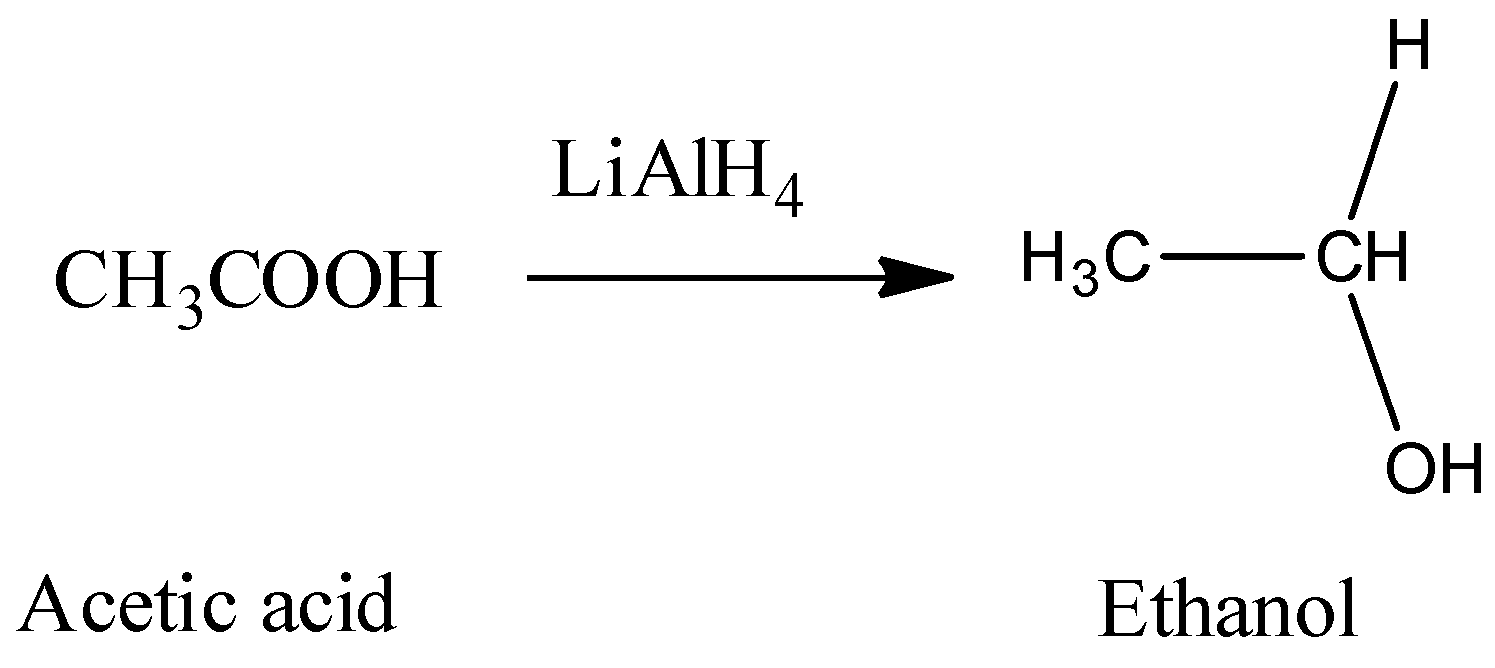
Hence, from the above reaction, we can see that acetone forms 3-propanol, Propanaldehyle forms 1-propanol and Acetic acid and acetaldehyde will react with lithium hydride to produce ethanol.
- So, only the compound that gives carbon dioxide with sodium bicarbonate and gives ethanol with lithium aluminum hydride is Acetic acid.
So, correct answer is (D) \[C{H_3}COOH\].
Note:
Remember that hydrogen atoms of aldehydes are not acidic and they cannot react with sodium bicarbonate to give carbon dioxide gas. Remember that lithium aluminum hydride converts –CHO and –COOH groups to \[ - C{H_2}OH\] groups.
Lithium aluminum hydride reacts with carbonyl carbon to give hydroxyl group in product. Sodium bicarbonate is a base and it reacts with acids to give salts. Both aldehydes and carboxylic acid groups will get reduced by lithium aluminum hydride.
Complete step by step answer:
It is proved from experiments that organic acids produce carbon dioxide gas when they react with sodium bicarbonate solution. So, salt of acid and carbon dioxide gas are produced as products. The release of carbon dioxide gas gives brisk effervescence. Let’s see which of the given compounds will give brisk effervescence when treated with sodium bicarbonate.
- \[C{H_3}COC{H_3}\] contains only ketone functional group, \[C{H_3}C{H_2}CHO\] and \[C{H_3}CHO\] contains aldehyde functional group. So, these three compounds will not react with sodium bicarbonate.
- \[C{H_3}COOH\] has a carboxylic acid group and is an organic acid, so it will react with sodium bicarbonate to produce brisk effervescence of carbon dioxide gas. The reaction is given as below.
\[\mathop {C{H_3}COOH}\limits_{{\text{Acetic acid}}} + NaHC{O_3} \to \mathop {C{H_3}COONa}\limits_{Sodium{\text{ Acetate}}} + C{O_2}\]
Now, Lithium aluminum hydride is a reducing agent and will reduce any carbonyl carbon to give hydroxyl group on it.
Let’s see how the given compounds will react with Lithium aluminum hydride.




Hence, from the above reaction, we can see that acetone forms 3-propanol, Propanaldehyle forms 1-propanol and Acetic acid and acetaldehyde will react with lithium hydride to produce ethanol.
- So, only the compound that gives carbon dioxide with sodium bicarbonate and gives ethanol with lithium aluminum hydride is Acetic acid.
So, correct answer is (D) \[C{H_3}COOH\].
Note:
Remember that hydrogen atoms of aldehydes are not acidic and they cannot react with sodium bicarbonate to give carbon dioxide gas. Remember that lithium aluminum hydride converts –CHO and –COOH groups to \[ - C{H_2}OH\] groups.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




