
The correct diagram representing the basic structure of an atom is:
(A)
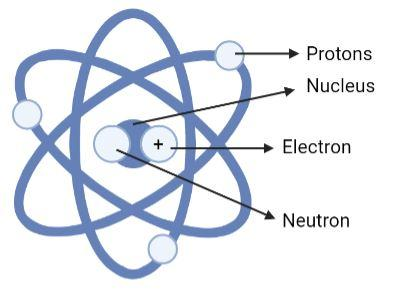
(B)
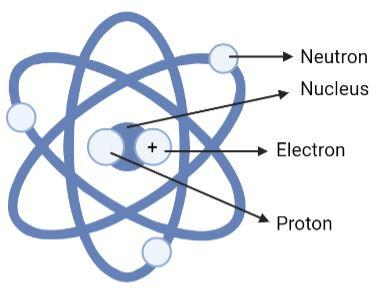
(C)
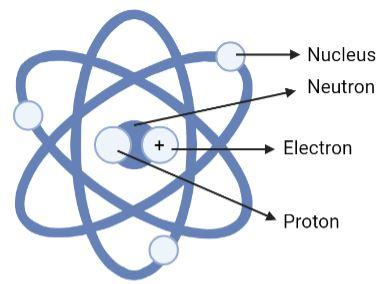
(D)





Answer
515.1k+ views
Hint: Atoms are represented as the fundamental building units of any matter. It is the smallest component that makes up matter which possesses various chemical elemental properties. Atoms do not live in isolation; instead, they merge to create molecules or ions, which then combine in large quantities to construct the different sources of matter we are familiar with.
Complete answer:
From all of the diagrams provided we see that the parts are labelled as electron, proton, neutron and nucleus. Once we are able to identify the position where each constituent particle must be present, we can find the correct diagram.
Atomic structure can be defined as the configuration of an atom that consists of a nucleus (present at the center) containing protons (particles that are charged positively) and neutrons (neutral charged particles). Electrons, which are negatively charged particles, are the particles that revolve about the central nucleus.
Now that we have a basic idea about where each particle must lie we can observe the diagrammatic representation of the atomic structure in each option to identify the correct positions:
Option (A) is an incorrect option. This is because in this diagram we see that the protons are revolving around the nucleus on the axes, and the positively indicated particle is labelled as electrons. Since positively charged particles can only be named as protons and the particles that revolve around the nucleus have to be electrons, this option can be rejected.
Option (B) is an incorrect option. This is because we see that the neutron, electron and proton are wrongly labelled in this diagram. The positively charged particle is proton not electron, the particle with no charge must be neutron not proton and the particles revolving around must be electron not neutron.
Option (C) is an incorrect option. This is wrong because all the particles are labelled in a wrong manner. The central part is not neutron it is the nucleus, then the positive particle is not electron but it is the proton, the particle revolving on the axes is not the nucleus but it is the electron and the part labelled as proton is actually a neutral particle the neutron.
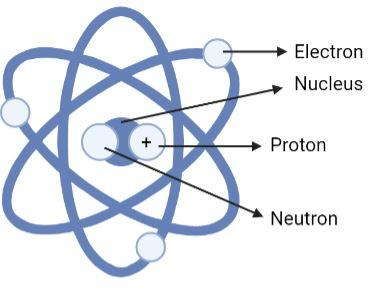
Option (D) is the correct option. In this diagram all the parts are labelled correctly, that is, the electron is revolving on the axes, the proton is the positively charged particle, the neutral charged particle is the neutron and the centermost part of the atom is the nucleus.
So we have evidently proved that the options (A), (B) and (C) have incorrect representations for the basic structure of an atom. But option (D) represents the structure of the atom perfectly so we may accept this option.
Therefore the correct answer is clearly option (D) with the following diagram:
Note:
An atom is simply the tiniest unit of an element that may or may not exist on their own. On the other hand, molecules are the simplest part or unit in a compound and are composed of a number of atoms that join together. As an example we can consider ozone, remember that three oxygen atoms can combine to form ozone and so it is known to be a molecule. The constituents of ozone are said to be the oxygen atoms.
Complete answer:
From all of the diagrams provided we see that the parts are labelled as electron, proton, neutron and nucleus. Once we are able to identify the position where each constituent particle must be present, we can find the correct diagram.
Atomic structure can be defined as the configuration of an atom that consists of a nucleus (present at the center) containing protons (particles that are charged positively) and neutrons (neutral charged particles). Electrons, which are negatively charged particles, are the particles that revolve about the central nucleus.
Now that we have a basic idea about where each particle must lie we can observe the diagrammatic representation of the atomic structure in each option to identify the correct positions:
Option (A) is an incorrect option. This is because in this diagram we see that the protons are revolving around the nucleus on the axes, and the positively indicated particle is labelled as electrons. Since positively charged particles can only be named as protons and the particles that revolve around the nucleus have to be electrons, this option can be rejected.
Option (B) is an incorrect option. This is because we see that the neutron, electron and proton are wrongly labelled in this diagram. The positively charged particle is proton not electron, the particle with no charge must be neutron not proton and the particles revolving around must be electron not neutron.
Option (C) is an incorrect option. This is wrong because all the particles are labelled in a wrong manner. The central part is not neutron it is the nucleus, then the positive particle is not electron but it is the proton, the particle revolving on the axes is not the nucleus but it is the electron and the part labelled as proton is actually a neutral particle the neutron.
Option (D) is the correct option. In this diagram all the parts are labelled correctly, that is, the electron is revolving on the axes, the proton is the positively charged particle, the neutral charged particle is the neutron and the centermost part of the atom is the nucleus.
So we have evidently proved that the options (A), (B) and (C) have incorrect representations for the basic structure of an atom. But option (D) represents the structure of the atom perfectly so we may accept this option.
Therefore the correct answer is clearly option (D) with the following diagram:

Note:
An atom is simply the tiniest unit of an element that may or may not exist on their own. On the other hand, molecules are the simplest part or unit in a compound and are composed of a number of atoms that join together. As an example we can consider ozone, remember that three oxygen atoms can combine to form ozone and so it is known to be a molecule. The constituents of ozone are said to be the oxygen atoms.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




