
The correct IUPAC name of the given compound is:
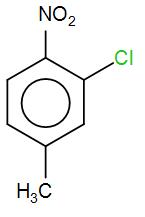
[A] 5-chloro-4-methyl-1-nitrobenzene
[B] 2-methyl-5-nitro-1chlorobenzene
[C] 3-chloro-4-methyl-1-nitrobenzene
[D] 2-chloro-1-methyl-4-nitrobenzene

Answer
578.7k+ views
HINT: IUPAC is the world-wide accepted naming system. To find out the IUPAC name of the given compound, start by naming the carbon atoms in the ring. Remember the priority order for nomenclature.
COMPLETE STEP BY STEP SOLUTION: We know that IUPAC nomenclature is a method of naming chemical compounds as recommended by the International Union of Pure and Applied Chemistry. There are certain rules that we need to follow while writing down the IUPAC name of compounds. Firstly, we need to see the number of carbon atoms. Here, we have 6 carbon atoms forming an aromatic ring with alternate double bonds which is also known as benzene. As we can see that the compound given to us is a derivative of benzene. In IUPAC we name a system by naming the substituent on the particular carbon atom instead of using Ortho, Meta and Para prefix. Here, let us number the carbon atoms first and then try to find out the IUPAC name. We need to be careful while numbering the carbon atoms. Higher priority is given to the methyl group followed by chlorine and then the nitro group. So, we’ve numbered it accordingly as-
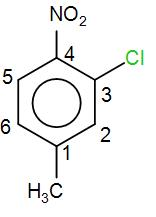
We can see that we have methyl at ${{C}_{1}}$ so we will have 1-methyl. Next, we have chlorine at ${{C}_{3}}$ so we have 3-chloro and lastly we have nitro group at ${{C}_{4}}$ so we will have 4-nitro. Now, we will arrange these accordingly propriety wise and the name will end with the prefix benzene. So, the IUPAC name will come out to be - 2-chloro-1-methyl-4-nitrobenzene
Therefore, the correct answer is option [D] 2-chloro-1-methyl-4-nitrobenzene.
NOTE: The International Union of Pure and Applied Chemistry, abbreviated as IUPAC relies on the nomenclature of various benzene derived compounds on the simple benzene naming system therefore, Ortho, Meta and Para terms are not accepted by the IUPAC. Compounds with Ortho, Meta or Para substitutions are named according to the common benzene system and can be converted to systematic names. For example, aniline is the common preferred IUPAC name and its systematic IUPAC name is benzamine. Ortho-nitroaniline is the common name for 2-Nitronianiline, Meta-nitroaniline is 3-Nitroaniline and Para-nitroaniline is 4-Nitroaniline.
COMPLETE STEP BY STEP SOLUTION: We know that IUPAC nomenclature is a method of naming chemical compounds as recommended by the International Union of Pure and Applied Chemistry. There are certain rules that we need to follow while writing down the IUPAC name of compounds. Firstly, we need to see the number of carbon atoms. Here, we have 6 carbon atoms forming an aromatic ring with alternate double bonds which is also known as benzene. As we can see that the compound given to us is a derivative of benzene. In IUPAC we name a system by naming the substituent on the particular carbon atom instead of using Ortho, Meta and Para prefix. Here, let us number the carbon atoms first and then try to find out the IUPAC name. We need to be careful while numbering the carbon atoms. Higher priority is given to the methyl group followed by chlorine and then the nitro group. So, we’ve numbered it accordingly as-

We can see that we have methyl at ${{C}_{1}}$ so we will have 1-methyl. Next, we have chlorine at ${{C}_{3}}$ so we have 3-chloro and lastly we have nitro group at ${{C}_{4}}$ so we will have 4-nitro. Now, we will arrange these accordingly propriety wise and the name will end with the prefix benzene. So, the IUPAC name will come out to be - 2-chloro-1-methyl-4-nitrobenzene
Therefore, the correct answer is option [D] 2-chloro-1-methyl-4-nitrobenzene.
NOTE: The International Union of Pure and Applied Chemistry, abbreviated as IUPAC relies on the nomenclature of various benzene derived compounds on the simple benzene naming system therefore, Ortho, Meta and Para terms are not accepted by the IUPAC. Compounds with Ortho, Meta or Para substitutions are named according to the common benzene system and can be converted to systematic names. For example, aniline is the common preferred IUPAC name and its systematic IUPAC name is benzamine. Ortho-nitroaniline is the common name for 2-Nitronianiline, Meta-nitroaniline is 3-Nitroaniline and Para-nitroaniline is 4-Nitroaniline.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




