
The correct IUPAC name of the structure is:
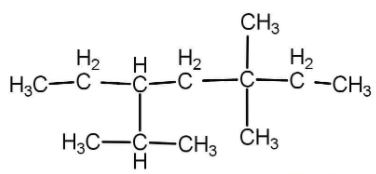
A) 3-isopropyl-5,5-dimethyl heptane
B) 5-Ethyl-3,3,6-trimethyl heptane
C) 3,3-Dimethyl-5-isopropyl heptane
D) 3-Ethyl-2,5,5-trimethyl heptane

Answer
548.4k+ views
Hint:
To write the name we have to follow the IUPAC nomenclature. First of all we need to select the straight chain of longest carbon. While writing the names, side substituents are written first and then the name.
Complete step by step solution:
First of all we need to select a parent chain that contains the maximum number of carbons. The chain must be a straight chain and no branching should be there. In whatsoever manner we select, in the above question we will get a straight chain of maximum 7 carbon atoms and has all single bonds. The name of the parent chain will be heptane.
Now we will select that carbon chain which has maximum number of side substituent and that is:
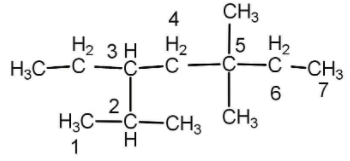
The numbering is done according to that the side substituents get maximum number. If we will start numbering from right then we will get the number 2234 and if we start numbering from left then we will get the number 2355. Hence we will start with the left.
There are 3 methyl groups as substituent and 1 ethyl group. Naming of substituent is done alphabetically so ethyl will be written first.
Ethyl group is on carbon number 3, methyl groups are in carbon number 2, 5 and 5 and there are trees so we will use tri as a prefix. Hence the name will be:
3-Ethyl-2,5,5-trimethyl heptanes
Thus, the correct option is D.
Note:
The IUPAC stands for International Union for Pure and Applied Chemistry. The IUPAC has set some norms for naming the organic compounds so that the standard names can be used worldwide and they are universally accepted.
To write the name we have to follow the IUPAC nomenclature. First of all we need to select the straight chain of longest carbon. While writing the names, side substituents are written first and then the name.
Complete step by step solution:
First of all we need to select a parent chain that contains the maximum number of carbons. The chain must be a straight chain and no branching should be there. In whatsoever manner we select, in the above question we will get a straight chain of maximum 7 carbon atoms and has all single bonds. The name of the parent chain will be heptane.
Now we will select that carbon chain which has maximum number of side substituent and that is:

The numbering is done according to that the side substituents get maximum number. If we will start numbering from right then we will get the number 2234 and if we start numbering from left then we will get the number 2355. Hence we will start with the left.
There are 3 methyl groups as substituent and 1 ethyl group. Naming of substituent is done alphabetically so ethyl will be written first.
Ethyl group is on carbon number 3, methyl groups are in carbon number 2, 5 and 5 and there are trees so we will use tri as a prefix. Hence the name will be:
3-Ethyl-2,5,5-trimethyl heptanes
Thus, the correct option is D.
Note:
The IUPAC stands for International Union for Pure and Applied Chemistry. The IUPAC has set some norms for naming the organic compounds so that the standard names can be used worldwide and they are universally accepted.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




