
The correct order of acidity of the following is:
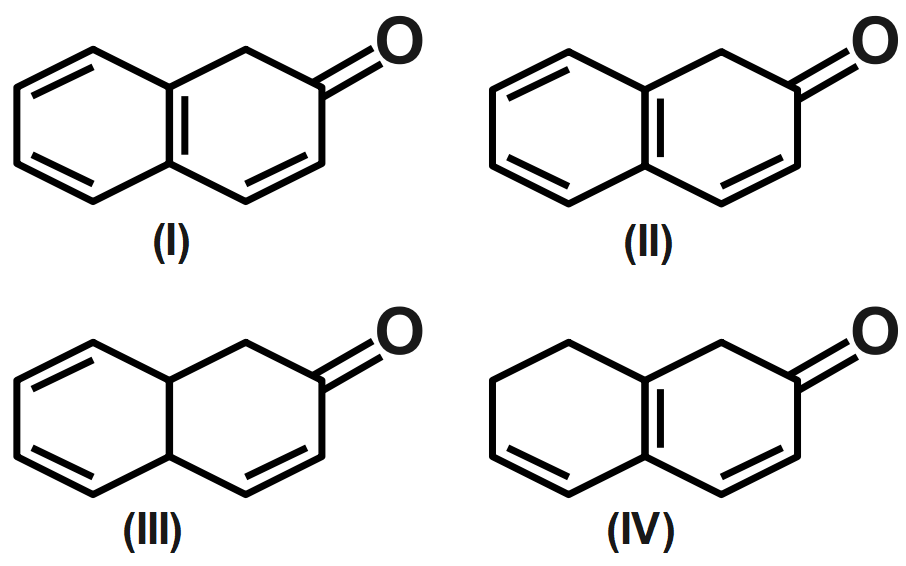
A. III < IV < I < II
B. III < IV < II < I
C. IV < III < I < II
D. None of these.

Answer
532.2k+ views
Hint: We know that the Electron donating group tends to decrease the acidic strength of phenols and electron withdrawing group tends to increase the acidic strength phenols. The more electron donating tendency any group has, the less acidic it will be with less acidic strength.
Complete step by step answer:
An acid is formed by the removal of hydrogen ions from a molecule. Now if the hydrogen ions are removed from phenol and phenoxide ion is formed which is an anion. The stability of this anion will determine whether the compound will be more acidic or less acidic. The more stable this negative charge will be, more will be the acidic character.
Electron donating groups tend to increase the electron density hence destabilising the negative charge and makes the molecule less acidic whereas the electron withdrawing makes the compound more acidic by withdrawing some negative charge from the molecule and stabilises it.
Methyl donates electrons and shows inductive effect whereas chloride, sulphide and nitro groups are electron withdrawing groups. The third molecule will be least acidic because of the presence of an electron donating group that is methyl. Among chloride, sulphide and nitro groups, nitro groups withdraw electrons through resonance whereas in case of halogen or halide minus I effect dominates.
So, the correct answer is Option A.
Note: If any functional group tends to donate electrons or withdraw electrons by resonance such effect. The group which donates the electrons to the carbon chain through resonance are known as $+M$ groups and the group or atoms which withdraw electrons from the carbon chain.
Complete step by step answer:
An acid is formed by the removal of hydrogen ions from a molecule. Now if the hydrogen ions are removed from phenol and phenoxide ion is formed which is an anion. The stability of this anion will determine whether the compound will be more acidic or less acidic. The more stable this negative charge will be, more will be the acidic character.
Electron donating groups tend to increase the electron density hence destabilising the negative charge and makes the molecule less acidic whereas the electron withdrawing makes the compound more acidic by withdrawing some negative charge from the molecule and stabilises it.
Methyl donates electrons and shows inductive effect whereas chloride, sulphide and nitro groups are electron withdrawing groups. The third molecule will be least acidic because of the presence of an electron donating group that is methyl. Among chloride, sulphide and nitro groups, nitro groups withdraw electrons through resonance whereas in case of halogen or halide minus I effect dominates.
So, the correct answer is Option A.
Note: If any functional group tends to donate electrons or withdraw electrons by resonance such effect. The group which donates the electrons to the carbon chain through resonance are known as $+M$ groups and the group or atoms which withdraw electrons from the carbon chain.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




