
The crystalline structure of \[NaCl\] is
A. Hexagonal close packing
B. Face-centred cubic
C. square planar
D. body-centred cubic
Answer
590.7k+ views
Hint: A unit cell that has a lattice point at every face centre in addition to the lattice point at every corner is called a face-centred cubic unit cell. In the case of \[NaCl\], the ratio of chloride to the ratio of sodium ions is 1:1.
Complete step by step answer:
In the case of \[NaCl\], the structure is a rock salt type structure with coordination number being in a ratio of 6:6 which means that there are 6 chloride ions present on the 6 faces of the face centred cubic system and 6 Na+ ions occupy the octahedral voids in the lattice system. There are many compounds that have a similar structure as the sodium chloride structure. The ratio of radius range of anions and cations ranges between 225-414 nm. Those structures which have a similar structure to that of \[NaCl\] include different alkyl halides, oxides of different metals, etc. In the case of a face-centred cubic system, the coordination number will be 12 and the distance between the two nearest atoms will be $\dfrac{{a\sqrt 2 }}{2}$ . The relation between the radius and side length in such a system is $r = \frac{a}{{2\sqrt 2 }}$ .
Thus, the correct option is (B) Face-centred cubic.
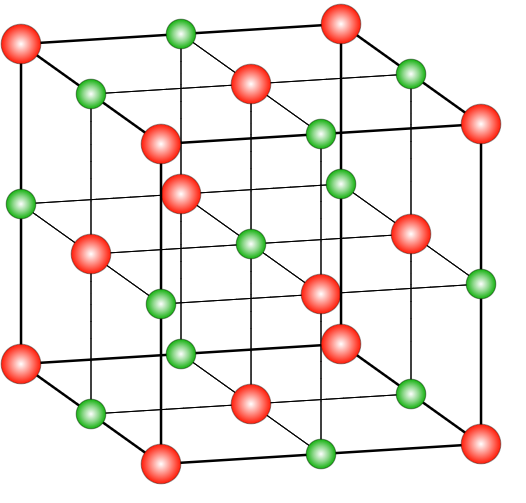
where red spheres represent $Cl^-$ and green ones are for $Na^+$
Note:
In a face centred cubic system, along the face diagonal, all the atoms touch each other and the length of the face diagonal is $a\sqrt 2 $ . The number of atoms per unit cell in FCC lattice is 4 and the packing efficiency is 74% with void space of 26%.
Complete step by step answer:
In the case of \[NaCl\], the structure is a rock salt type structure with coordination number being in a ratio of 6:6 which means that there are 6 chloride ions present on the 6 faces of the face centred cubic system and 6 Na+ ions occupy the octahedral voids in the lattice system. There are many compounds that have a similar structure as the sodium chloride structure. The ratio of radius range of anions and cations ranges between 225-414 nm. Those structures which have a similar structure to that of \[NaCl\] include different alkyl halides, oxides of different metals, etc. In the case of a face-centred cubic system, the coordination number will be 12 and the distance between the two nearest atoms will be $\dfrac{{a\sqrt 2 }}{2}$ . The relation between the radius and side length in such a system is $r = \frac{a}{{2\sqrt 2 }}$ .
Thus, the correct option is (B) Face-centred cubic.

where red spheres represent $Cl^-$ and green ones are for $Na^+$
Note:
In a face centred cubic system, along the face diagonal, all the atoms touch each other and the length of the face diagonal is $a\sqrt 2 $ . The number of atoms per unit cell in FCC lattice is 4 and the packing efficiency is 74% with void space of 26%.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




