
The density of phosphorus vapor at 310\[^{o}C\] and 775 torr is 2.64\[g\text{ }d{{m}^{-3}}\]. What is the molecular formula of phosphorus?
Answer
590.4k+ views
Hint: Using the density, we can find the ratio of mass by volume which will be used in the formula to calculate the molar mass. We can calculate the molar mass of gas vapor using the below formula.
PV= nRT
P = pressure, V = volume, R = gas constant, T = temperature
n = \[\dfrac{mass}{molar\text{ }mass}\]
Complete answer:
In the question it is given that density \[\rho \]= 2.64 \[g\text{ }d{{m}^{-3}}\]= 2.64 g/L
The pressure of the vapour P = 775 mm
Temperature of the phosphorus vapor = 310 +273 = 583 K
We have to convert pressure in mm into atm.
775 mm = \[\dfrac{775}{760}=1.02atm\]
Gas constant R = 0.0821 liter/atm. K. mol
Now substitute all the above formulas in the equation PV = nRT
\[\begin{align}
& PV=\dfrac{mass}{molar\text{ }mass}RT \\
& molarmass=\dfrac{mass}{P\times V}RT \\
\end{align}\]
We know that density = \[\dfrac{mass}{volume}\]
Therefore molar mass = \[\dfrac{\rho }{P}RT\](where\[\rho \]= density)
\[\begin{align}
& =\dfrac{2.64\times 0.0821\times 583}{1.02} \\
& =123.88g \\
\end{align}\]
We know that Molar mass of the one phosphorus atom = 31 g
There is a formula to calculate the number of phosphorus atoms
Number of atoms
\[\begin{align}
& =\dfrac{molar\text{ }mass}{atomic\text{ }weight} \\
& =\dfrac{123.88}{31} \\
& =4 \\
\end{align}\]
That means there are four phosphorus atoms in the phosphorus molecule.
So, the molecular formula of phosphorus molecules is\[{{P}_{4}}\].
Note:
The structure of the white phosphorus molecule is as follows.
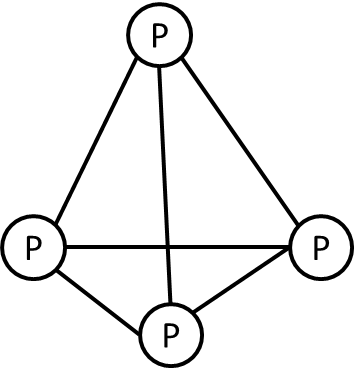
The phosphorus atoms are arranged in a tetrahedral structure.
Phosphorus molecules exist in two allotropic forms. One is white phosphorus and another is red phosphorus.
While solving this problem, remember to convert the pressure given here in mm to atm. Use the value of R which has the unit atm in it i.e 0.0821 liter/atm. K. mol. There are other values also for R, but since the pressure is converted here into atm, we have to use the above value.
PV= nRT
P = pressure, V = volume, R = gas constant, T = temperature
n = \[\dfrac{mass}{molar\text{ }mass}\]
Complete answer:
In the question it is given that density \[\rho \]= 2.64 \[g\text{ }d{{m}^{-3}}\]= 2.64 g/L
The pressure of the vapour P = 775 mm
Temperature of the phosphorus vapor = 310 +273 = 583 K
We have to convert pressure in mm into atm.
775 mm = \[\dfrac{775}{760}=1.02atm\]
Gas constant R = 0.0821 liter/atm. K. mol
Now substitute all the above formulas in the equation PV = nRT
\[\begin{align}
& PV=\dfrac{mass}{molar\text{ }mass}RT \\
& molarmass=\dfrac{mass}{P\times V}RT \\
\end{align}\]
We know that density = \[\dfrac{mass}{volume}\]
Therefore molar mass = \[\dfrac{\rho }{P}RT\](where\[\rho \]= density)
\[\begin{align}
& =\dfrac{2.64\times 0.0821\times 583}{1.02} \\
& =123.88g \\
\end{align}\]
We know that Molar mass of the one phosphorus atom = 31 g
There is a formula to calculate the number of phosphorus atoms
Number of atoms
\[\begin{align}
& =\dfrac{molar\text{ }mass}{atomic\text{ }weight} \\
& =\dfrac{123.88}{31} \\
& =4 \\
\end{align}\]
That means there are four phosphorus atoms in the phosphorus molecule.
So, the molecular formula of phosphorus molecules is\[{{P}_{4}}\].
Note:
The structure of the white phosphorus molecule is as follows.

The phosphorus atoms are arranged in a tetrahedral structure.
Phosphorus molecules exist in two allotropic forms. One is white phosphorus and another is red phosphorus.
While solving this problem, remember to convert the pressure given here in mm to atm. Use the value of R which has the unit atm in it i.e 0.0821 liter/atm. K. mol. There are other values also for R, but since the pressure is converted here into atm, we have to use the above value.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




