
The diagram represents the preparation of sodium sulphate salt from dilute ${H_2}S{O_4}$ acid and sodium hydroxide. Name the substance $X$ placed in $A$ and the substance $Y$ placed in $B$ .
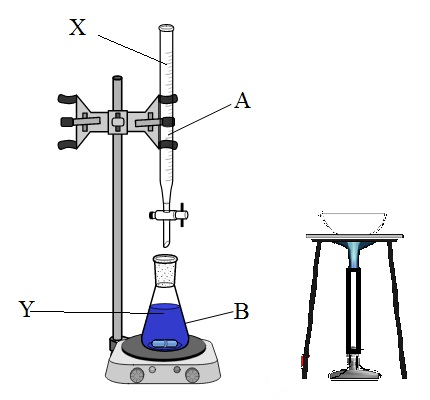
A.$X = NaOH,Y = dil{H_2}S{O_4}$
B.$X = dil{H_2}S{O_4},Y = NaOH$
C.$X = N{a_2}S{O_4},Y = NaOH$
D.None of the above

Answer
570k+ views
Hint:
The reaction given in the diagram is the acid-base reaction. Acid- base reaction is also called a neutralization reaction. In neutralization reaction acid and base react to form salt and water.
Complete answer:
The dilute sulphuric acid reacts with sodium hydroxide to form sodium sulphate salt and water respectively.
The reaction is given as follows:
$2NaOH + {H_2}S{O_4} \to N{a_2}S{O_4} + 2{H_2}O$
So during the titration experiment sulphuric acid is in the burette and sodium hydroxide is in the conical flask.
When dilute sulphuric acid is titrated with sodium hydroxide it neutralizes completely and the salt which is formed is sodium sulphate salt and the solution in it is just water.
We use phenolphthalein as an indicator in order to see if the reaction is complete or not.
When we add phenolphthalein to the sodium hydroxide solution it turns pink and during titration it changes its colour from pink to colorless.
This color change shows that the reaction is complete.
This type of reaction is known as neutralization reaction.
Neutralization reactions are reactions in which acid and base react with each other to give salt and water respectively.
In neutralization reactions equal amounts of hydrogen and hydroxide ions react with each other.
We always add acid to basic or neutral solution and not vice versa because if we add base or neutral solution to acid the given acid solution will become highly concentrated because of which it gets heated up and will splash out of the container causing harm to the surrounding.
So for this reason in the given experiment the sulphuric acid is added to sodium hydroxide solution in the conical flask.
Therefore, the correct answer is option B) $X = dil{H_2}S{O_4},Y = NaOH$
Note:Phenolphthalein indicator shows pink colour when the solution is basic and shows no color when the solution is acidic because the pH range of phenolphthalein indicator is between $8 - 10$ and also the basic range lies between $8 - 14$ . So that’s why phenolphthalein is used as an indicator for acid- base titrations.
The reaction given in the diagram is the acid-base reaction. Acid- base reaction is also called a neutralization reaction. In neutralization reaction acid and base react to form salt and water.
Complete answer:
The dilute sulphuric acid reacts with sodium hydroxide to form sodium sulphate salt and water respectively.
The reaction is given as follows:
$2NaOH + {H_2}S{O_4} \to N{a_2}S{O_4} + 2{H_2}O$
So during the titration experiment sulphuric acid is in the burette and sodium hydroxide is in the conical flask.
When dilute sulphuric acid is titrated with sodium hydroxide it neutralizes completely and the salt which is formed is sodium sulphate salt and the solution in it is just water.
We use phenolphthalein as an indicator in order to see if the reaction is complete or not.
When we add phenolphthalein to the sodium hydroxide solution it turns pink and during titration it changes its colour from pink to colorless.
This color change shows that the reaction is complete.
This type of reaction is known as neutralization reaction.
Neutralization reactions are reactions in which acid and base react with each other to give salt and water respectively.
In neutralization reactions equal amounts of hydrogen and hydroxide ions react with each other.
We always add acid to basic or neutral solution and not vice versa because if we add base or neutral solution to acid the given acid solution will become highly concentrated because of which it gets heated up and will splash out of the container causing harm to the surrounding.
So for this reason in the given experiment the sulphuric acid is added to sodium hydroxide solution in the conical flask.
Therefore, the correct answer is option B) $X = dil{H_2}S{O_4},Y = NaOH$
Note:Phenolphthalein indicator shows pink colour when the solution is basic and shows no color when the solution is acidic because the pH range of phenolphthalein indicator is between $8 - 10$ and also the basic range lies between $8 - 14$ . So that’s why phenolphthalein is used as an indicator for acid- base titrations.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




