
The fact about the Tyndall effect is:
(A) The diameter of dispersed particles is much smaller than the wavelength of light used
(B) refractive indices of the dispersed phase and dispersion medium differ greatly in magnitude
(C) it is shown by true solution
(D) all of the above
Answer
573.9k+ views
Hint: Tyndall effect is a phenomenon in which the particles scatter the beam of light .The Tyndall effect is shown by the solution containing colloidal particles. The effect is observed in the solution in which the matter is dispersed in a medium.
Complete Solution :
We have been asked about the conditions required to observe the Tyndall effect,
The phenomenon of scattering of light by colloidal particles as a result of which the path of the beam becomes visible is called the Tyndall effect.
The cause of the Tyndall effect is the scattering of light by the colloidal particles i.e. these particles scatter light in all directions in space. The scattering of light illuminates the path of the beam in the colloidal dispersion. The particles in the true solution are too small in size to cause any scattering i.e.tyndall effect is not observed in the true solution.
Let's consider the following illustration.
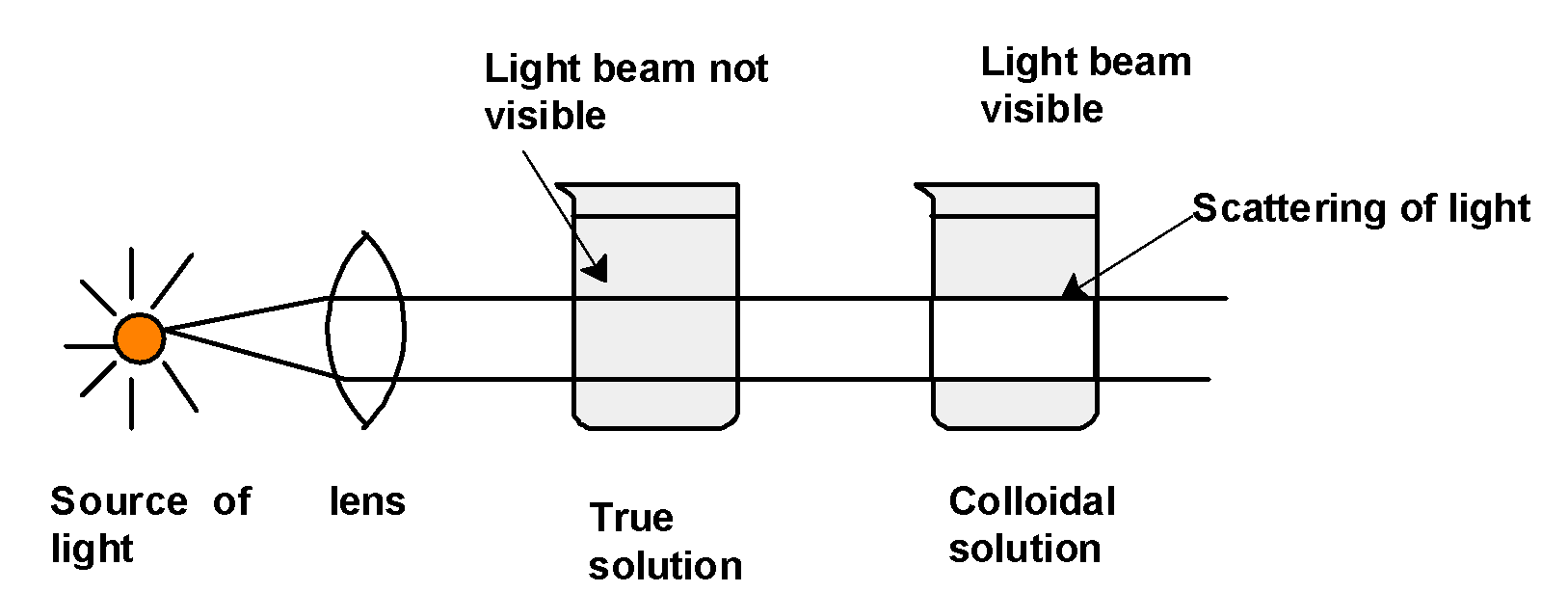
If the light is passed through a sol placed in the same room, the path of the light becomes visible when viewed from a direction at the right angle to that of the incident beam. The colloidal solutions appear reasonably clear or translucent by the transmitted light but they show a mild to strong opalescence when viewed at right angles to the passage of light i.e. the path of the beam gets illuminated by a bluish light.
The Tyndall effect is observed when the following two conditions are satisfied:
1) The diameter of the dispersed phase particles is not much smaller than the wavelength of the light used.
2) The refractive indices of the dispersed phase and the dispersion medium differ largely in magnitude.
So, the correct answer is “Option C”.
Note: Note that, Tyndall effect confirms the heterogeneous nature of the colloidal solution.it is observed due to the scattering of dust particles. Tyndall effect is used to distinguish between the true solution and the colloidal solution.
Complete Solution :
We have been asked about the conditions required to observe the Tyndall effect,
The phenomenon of scattering of light by colloidal particles as a result of which the path of the beam becomes visible is called the Tyndall effect.
The cause of the Tyndall effect is the scattering of light by the colloidal particles i.e. these particles scatter light in all directions in space. The scattering of light illuminates the path of the beam in the colloidal dispersion. The particles in the true solution are too small in size to cause any scattering i.e.tyndall effect is not observed in the true solution.
Let's consider the following illustration.

If the light is passed through a sol placed in the same room, the path of the light becomes visible when viewed from a direction at the right angle to that of the incident beam. The colloidal solutions appear reasonably clear or translucent by the transmitted light but they show a mild to strong opalescence when viewed at right angles to the passage of light i.e. the path of the beam gets illuminated by a bluish light.
The Tyndall effect is observed when the following two conditions are satisfied:
1) The diameter of the dispersed phase particles is not much smaller than the wavelength of the light used.
2) The refractive indices of the dispersed phase and the dispersion medium differ largely in magnitude.
So, the correct answer is “Option C”.
Note: Note that, Tyndall effect confirms the heterogeneous nature of the colloidal solution.it is observed due to the scattering of dust particles. Tyndall effect is used to distinguish between the true solution and the colloidal solution.
Recently Updated Pages
Master Class 9 General Knowledge: Engaging Questions & Answers for Success

Master Class 9 Social Science: Engaging Questions & Answers for Success

Master Class 9 English: Engaging Questions & Answers for Success

Master Class 9 Maths: Engaging Questions & Answers for Success

Master Class 9 Science: Engaging Questions & Answers for Success

Class 9 Question and Answer - Your Ultimate Solutions Guide

Trending doubts
Difference Between Plant Cell and Animal Cell

Fill the blanks with the suitable prepositions 1 The class 9 english CBSE

Who is eligible for RTE class 9 social science CBSE

Which places in India experience sunrise first and class 9 social science CBSE

What is pollution? How many types of pollution? Define it

Name 10 Living and Non living things class 9 biology CBSE




