
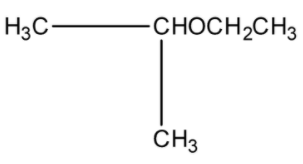
The final product (IV) in the sequence of the reaction is:
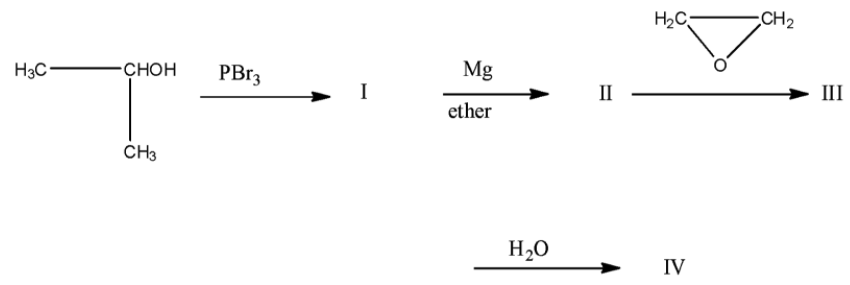
(a)-
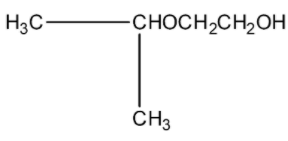
(b)-
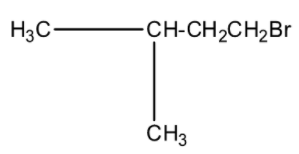
(c)-
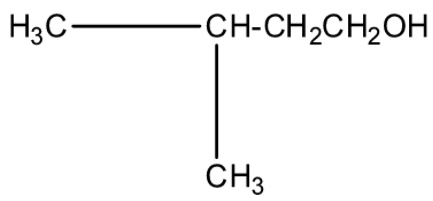
(d)-





Answer
560.7k+ views
Hint: The reactant in the question is isopropanol or its IUPAC name is propan-2-ol. When this reacts with phosphorus tribromide, then the alcohol group will be replaced with the bromide ion. Product II is reacted with oxirane, and then the epoxide ring will open.
Complete step by step answer:
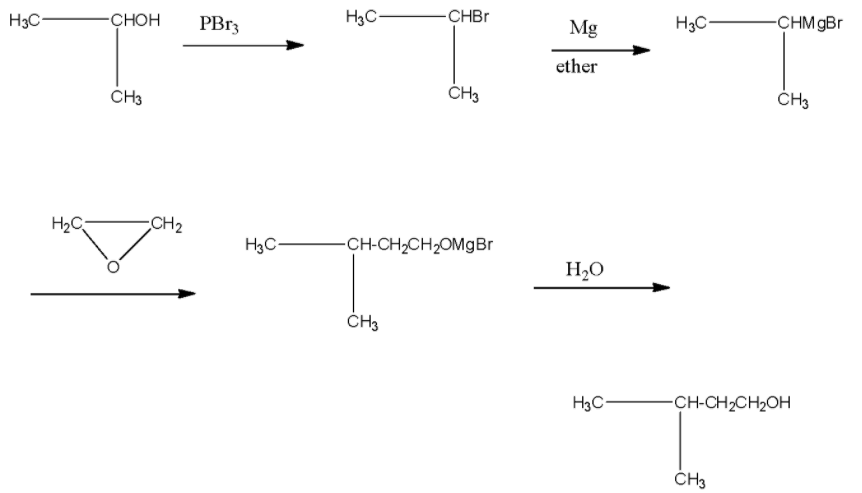
- The reactant in the question is isopropanol or its IUPAC name is propan-2-ol. In the first reaction, the isopropanol is reacted with phosphorus tribromide, and then the alcohol group present at the second carbon atom will be replaced with the bromine atom. The product I will be is isopropyl bromide or it is known as 2-Bromopropane.
- Now, this 2-Bromopropane is treated with magnesium in the presence of ether, this will lead to the formation of a Grignard reagent, i.e., the magnesium will be attached with the bromine atom. Product II will be isopropyl magnesium bromide.
This isopropyl magnesium bromide is treated with oxirane, and then the epoxide ring will open. This opened ring will attach between the carbon and magnesium atom. Now, when this compound is hydrolyzed or when this compound is treated with water then the magnesium bromide will be replaced with a hydrogen atom. The product will be 2-methyl butanol. The products series wise is given below:
The correct answer is option “C” .
Note: When any alkyl halide is treated with water then all the halide atoms present in the compound will be hydrolyzed to form alcohol. The oxirane ring is introduced in the compound to increase the number of carbon atoms in the chain.
Complete step by step answer:
- The reactant in the question is isopropanol or its IUPAC name is propan-2-ol. In the first reaction, the isopropanol is reacted with phosphorus tribromide, and then the alcohol group present at the second carbon atom will be replaced with the bromine atom. The product I will be is isopropyl bromide or it is known as 2-Bromopropane.
- Now, this 2-Bromopropane is treated with magnesium in the presence of ether, this will lead to the formation of a Grignard reagent, i.e., the magnesium will be attached with the bromine atom. Product II will be isopropyl magnesium bromide.
This isopropyl magnesium bromide is treated with oxirane, and then the epoxide ring will open. This opened ring will attach between the carbon and magnesium atom. Now, when this compound is hydrolyzed or when this compound is treated with water then the magnesium bromide will be replaced with a hydrogen atom. The product will be 2-methyl butanol. The products series wise is given below:

The correct answer is option “C” .
Note: When any alkyl halide is treated with water then all the halide atoms present in the compound will be hydrolyzed to form alcohol. The oxirane ring is introduced in the compound to increase the number of carbon atoms in the chain.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




