
The formation of phenol from benzene diazonium chloride is a -------.
A. Hydrolysis reaction
B. Pyrolysis reaction
C. Photosynthesis reaction
D. Combustion reaction
Answer
588k+ views
Hint: The benzene diazonium chloride is going to be prepared from aniline by the reaction with hydrochloric acid and sodium nitrate.
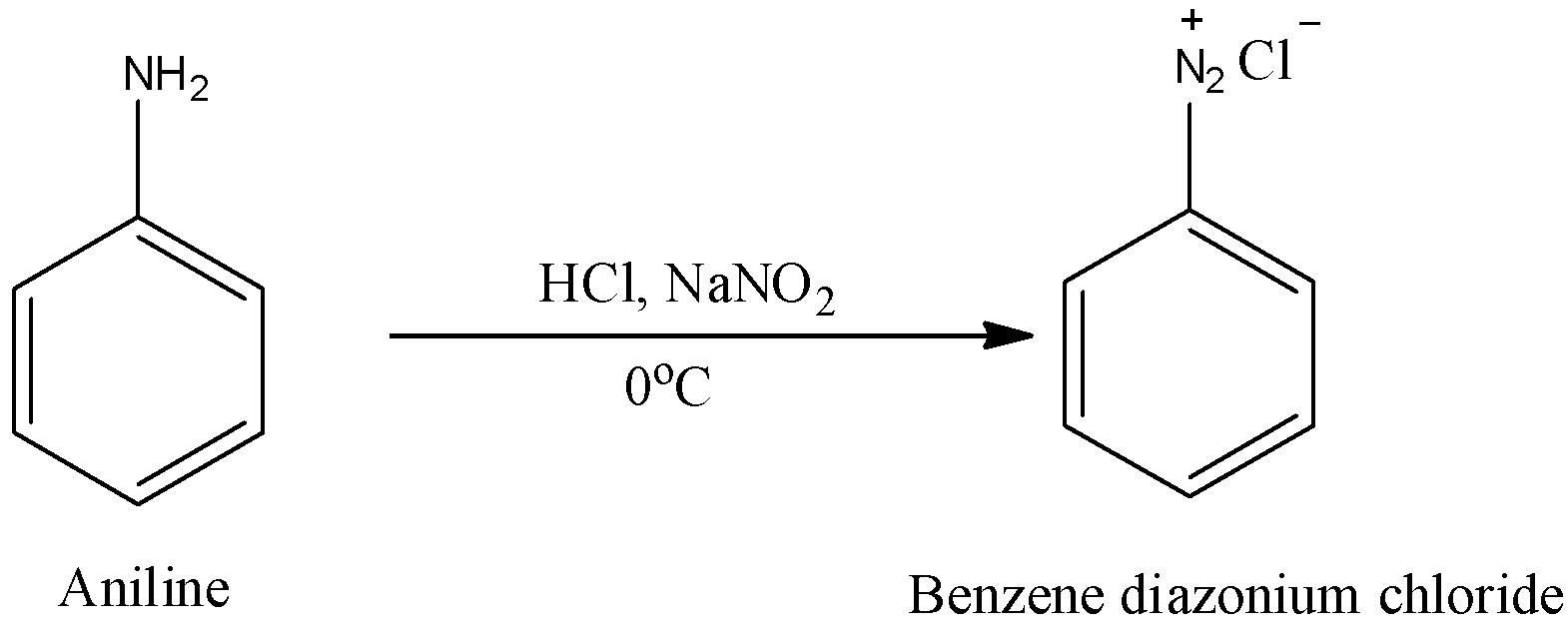
Benzene diazonium chloride plays a big role in the synthesis of a variety of compounds where few substituents cannot be added directly on the aromatic ring through electrophilic substitution reactions.
Complete step by step answer:
- In the question it is given that phenol is prepared from benzene diazonium chloride. We have to find which type of reaction benzene diazonium chloride undergoes to form phenol.
- Benzene diazonium chloride on heating in presence of aqueous solution gives phenol as a main product and nitrogen gas a byproduct.
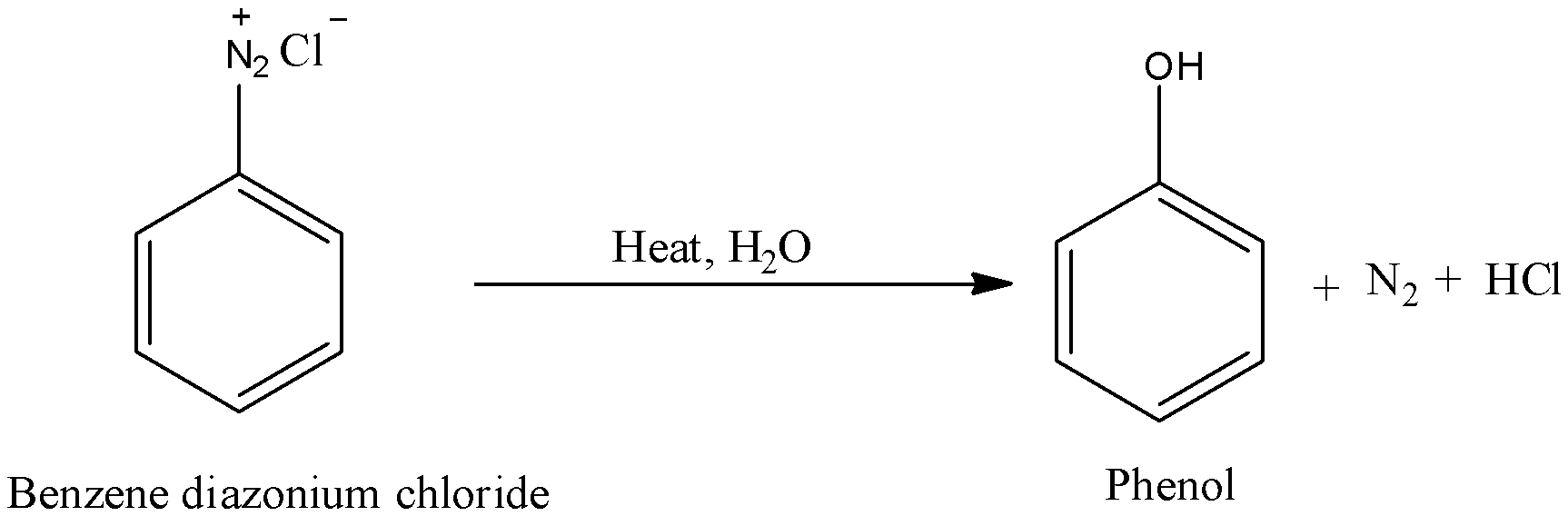
- Benzene diazonium chloride is hydrolyzed to phenols by treating it with dilute acids.
- Therefore the preparation of phenol from benzene diazonium chloride involves hydrolysis reaction.
- So, the correct option is A.
Additional information:
- Benzene diazonium chloride is ionic in nature (contains positive charge on nitrogen and a negative charge on chlorine).
- Benzene diazonium chloride is water-soluble.
- Benzene diazonium chloride is colorless and crystalline solid.
- Benzene diazonium chloride is soluble in water and reacts only when it is heated.
Note: Benzene diazonium chloride is useful in the synthesis of a variety of organic compounds particularly aryl derivatives. Benzene diazonium chloride used as an intermediate for introducing Fluorine, Bromo, Chloro and Iodo groups on the aromatic ring.

Benzene diazonium chloride plays a big role in the synthesis of a variety of compounds where few substituents cannot be added directly on the aromatic ring through electrophilic substitution reactions.
Complete step by step answer:
- In the question it is given that phenol is prepared from benzene diazonium chloride. We have to find which type of reaction benzene diazonium chloride undergoes to form phenol.
- Benzene diazonium chloride on heating in presence of aqueous solution gives phenol as a main product and nitrogen gas a byproduct.

- Benzene diazonium chloride is hydrolyzed to phenols by treating it with dilute acids.
- Therefore the preparation of phenol from benzene diazonium chloride involves hydrolysis reaction.
- So, the correct option is A.
Additional information:
- Benzene diazonium chloride is ionic in nature (contains positive charge on nitrogen and a negative charge on chlorine).
- Benzene diazonium chloride is water-soluble.
- Benzene diazonium chloride is colorless and crystalline solid.
- Benzene diazonium chloride is soluble in water and reacts only when it is heated.
Note: Benzene diazonium chloride is useful in the synthesis of a variety of organic compounds particularly aryl derivatives. Benzene diazonium chloride used as an intermediate for introducing Fluorine, Bromo, Chloro and Iodo groups on the aromatic ring.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




