
The gas liberated by heating potassium permanganate (\[KMn{{O}_{4}}\]) is?
(A) Hydrogen
(B) Oxygen
(C) Nitrogen
(D) Methane
Answer
587.4k+ views
Hint: Whenever we are heating any chemical then the chemical converts from one form to other forms by liberating some gas. Potassium permanganate (\[KMn{{O}_{4}}\]) is a chemical that exists in the solid-state at room temperature.
Complete step by step solution:
-The structure of the potassium permanganate is as follows.
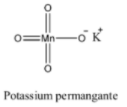
-The reaction of heating of potassium permanganate is as follows.
\[KMn{{O}_{4}}\xrightarrow{\Delta }{{K}_{2}}Mn{{O}_{4}}+Mn{{O}_{2}}+{{O}_{2}}\]
-On heating, potassium permanganate gives potassium manganate, Manganese dioxide and oxygen as the products.
-Coming to given options, option A Hydrogen. In the molecular formula of potassium permanganate (\[KMn{{O}_{4}}\]), there is no hydrogen. So, on heating potassium permanganate, hydrogen gas is not going to form as one of the product. Hence option A is wrong.
-Coming option B, Oxygen. In the molecular formula of potassium permanganate (\[KMn{{O}_{4}}\]), there is a presence of oxygen. So, on heating potassium permanganate, oxygen gas is going to form as one of the product. Hence option B is correct.
-Coming to option C, Nitrogen. In the molecular formula of potassium permanganate (\[KMn{{O}_{4}}\]), there is no nitrogen. So, on heating potassium permanganate, Nitrogen gas is not going to form as one of the product. Hence option C is wrong.
-Coming to option D, Methane. In the molecular formula of potassium permanganate (\[KMn{{O}_{4}}\]) there is no Carbon and hydrogen. So, on heating potassium permanganate, Methane gas is not going to form as one of the product. Hence option D is wrong.
-Therefore on heating potassium permanganate, oxygen gas is going to form as one of the product.
So, the correct option is (B).
Note: The oxidation state of Manganese in potassium permanganate (\[KMn{{O}_{4}}\]) is +7. On heating potassium permanganate (\[KMn{{O}_{4}}\]) gives potassium manganate (\[{{K}_{2}}Mn{{O}_{4}}\]). The oxidation state of manganese in \[{{K}_{2}}Mn{{O}_{4}}\] is +6.
Complete step by step solution:
-The structure of the potassium permanganate is as follows.

-The reaction of heating of potassium permanganate is as follows.
\[KMn{{O}_{4}}\xrightarrow{\Delta }{{K}_{2}}Mn{{O}_{4}}+Mn{{O}_{2}}+{{O}_{2}}\]
-On heating, potassium permanganate gives potassium manganate, Manganese dioxide and oxygen as the products.
-Coming to given options, option A Hydrogen. In the molecular formula of potassium permanganate (\[KMn{{O}_{4}}\]), there is no hydrogen. So, on heating potassium permanganate, hydrogen gas is not going to form as one of the product. Hence option A is wrong.
-Coming option B, Oxygen. In the molecular formula of potassium permanganate (\[KMn{{O}_{4}}\]), there is a presence of oxygen. So, on heating potassium permanganate, oxygen gas is going to form as one of the product. Hence option B is correct.
-Coming to option C, Nitrogen. In the molecular formula of potassium permanganate (\[KMn{{O}_{4}}\]), there is no nitrogen. So, on heating potassium permanganate, Nitrogen gas is not going to form as one of the product. Hence option C is wrong.
-Coming to option D, Methane. In the molecular formula of potassium permanganate (\[KMn{{O}_{4}}\]) there is no Carbon and hydrogen. So, on heating potassium permanganate, Methane gas is not going to form as one of the product. Hence option D is wrong.
-Therefore on heating potassium permanganate, oxygen gas is going to form as one of the product.
So, the correct option is (B).
Note: The oxidation state of Manganese in potassium permanganate (\[KMn{{O}_{4}}\]) is +7. On heating potassium permanganate (\[KMn{{O}_{4}}\]) gives potassium manganate (\[{{K}_{2}}Mn{{O}_{4}}\]). The oxidation state of manganese in \[{{K}_{2}}Mn{{O}_{4}}\] is +6.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

The Equation xxx + 2 is Satisfied when x is Equal to Class 10 Maths

Which Country is Called "The Land of Festivals"?

What is Contraception List its four different methods class 10 biology CBSE




