
The ${H_a}$ -line of hydrogen
(A) has a wavelength $4860\,\mathop A\limits^0 $
(B) has a wavelength $6562\,\mathop A\limits^0 $
(C) has a wavelength smaller than that of the ${H_\beta }$ -line
(D) is emitted in the transition from the second excited state of the first excited state.
Answer
577.5k+ views
Hint:The Balmer series includes the transition of the electrons from the higher energy levels like three or greater than three to the second energy level. If the transition takes place to the first energy level, it is included in the Lyman’s series.
Complete step by step solution:
The ${H_\alpha }$ is the visible deep red spectral line that is formed in the Balmer series when the hydrogen electron moves from the third to the second lowest energy level. It is considered as the brightest spectral line of the hydrogen that is visible. The wavelength of the ${H_\alpha }$ spectral line is $6562\,\mathop A\limits^0 $.
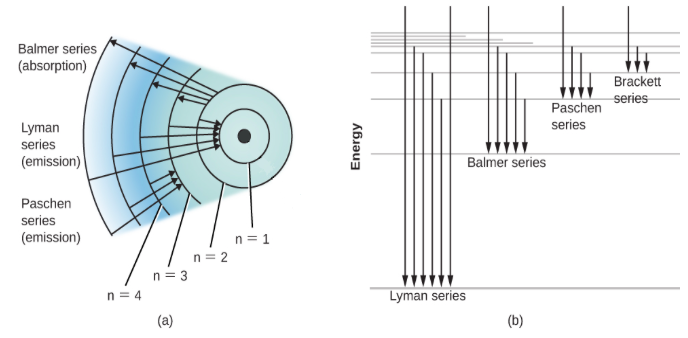
These molecules form the greater part of the nebulae and the clouds. The clouds are made up of the carbon dioxide, water molecules, ${H_\alpha }$ molecules, ammonia, formaldehyde etc. ${H_\alpha }$ helps in indicating the shape and the extent of the clouds and the other molecules help in forming the whole mass of the clouds. They have the wavelength greater than that of the ${H_\beta }$ line.
Thus the option (B) is correct.
Note:This ${H_\alpha }$ spectral line is more important since they are used to observe the sun’s atmosphere which includes the chromosphere and the solar prominence. By using this spectrum, the scientist and the astronomers can find the ionized hydrogen molecules present in the gas clouds.
Complete step by step solution:
The ${H_\alpha }$ is the visible deep red spectral line that is formed in the Balmer series when the hydrogen electron moves from the third to the second lowest energy level. It is considered as the brightest spectral line of the hydrogen that is visible. The wavelength of the ${H_\alpha }$ spectral line is $6562\,\mathop A\limits^0 $.

These molecules form the greater part of the nebulae and the clouds. The clouds are made up of the carbon dioxide, water molecules, ${H_\alpha }$ molecules, ammonia, formaldehyde etc. ${H_\alpha }$ helps in indicating the shape and the extent of the clouds and the other molecules help in forming the whole mass of the clouds. They have the wavelength greater than that of the ${H_\beta }$ line.
Thus the option (B) is correct.
Note:This ${H_\alpha }$ spectral line is more important since they are used to observe the sun’s atmosphere which includes the chromosphere and the solar prominence. By using this spectrum, the scientist and the astronomers can find the ionized hydrogen molecules present in the gas clouds.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




