
The hybridization and shape of $ Xe{F_2} $ is:
(A) $ s{p^3} $ , bent
(B) $ ds{p^2} $ , linear
(C) $ s{p^3}{d^2} $ , linear
(D) $ s{p^3}d $ , linear
Answer
560.7k+ views
Hint: We know that in chemistry hybridization or orbital hybridization is defined as the concept of mixing or merging atomic orbitals to form a new hybrid orbital which is also known as the hybridized orbital. With the help of hybridization, we can also predict the shape or the geometry of the molecule.
Complete step by step answer:
Now we know about the basic concept of hybridization in which two atomic orbitals are combined to form a hybrid orbital. So, now we will study the hybridization of the molecule $ Xe{F_2} $ . The hybridization of a molecule and the shape of a molecule depends on the central atom of the molecule. So, the central atom in the molecule $ Xe{F_2} $ is xenon $ Xe $ . Now we will write the electronic configuration of Xenon which is $ Xe \to [Kr]4{d^{10}}5{s^2}5{p^6} $ . The electronic configuration of the central atom helps us to find the number of valence electrons. So here according to the electronic configuration of Xenon, we can observe that it has a stable noble gas electronic configuration. So, the number of valence electrons in Xenon is $ 8 $ . Now we will study the chemical structure $ Xe{F_2} $ .
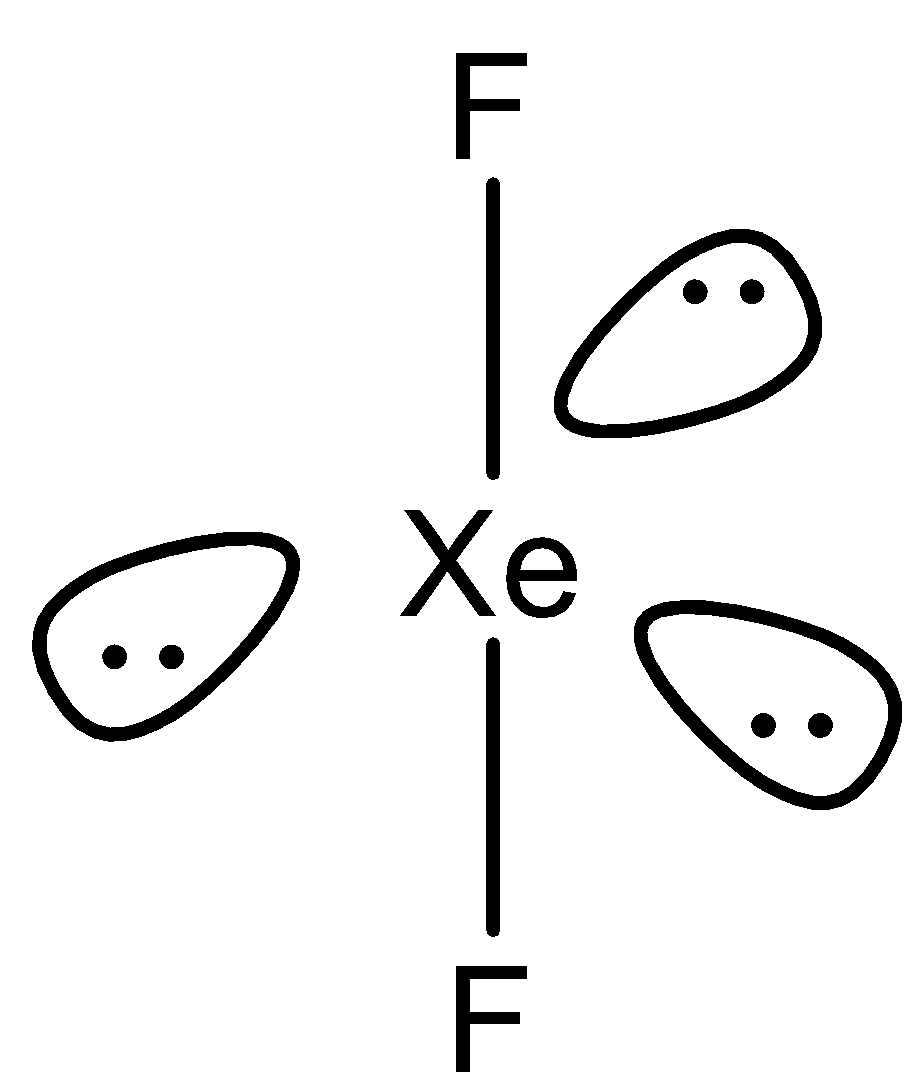
From the above structure we can observe that out of the $ 8 $ electrons, $ 2 $ electrons are bonded with fluorine atoms and the rest six electrons form three electron pairs. Now to predict the hybridization we count the sum of a total number of bonded atoms and the lone pairs over the central atom. So here, the sum is $ 5 $ . So, the hybridization $ Xe{F_2} $ will be $ s{p^3}d $ which means $ 1s $ , $ 3p $ and $ 2d $ orbitals are used. The shape $ Xe{F_2} $ is linear.
Therefore, the correct option is (D).
Note:
The shape of the molecule $ Xe{F_2} $ can be easily explained with the help of its structure. The shape is such that the lone pairs around the central atom acquire the equatorial position and the bond angle is $ {180^0} $ . Therefore, the shape is linear.
Complete step by step answer:
Now we know about the basic concept of hybridization in which two atomic orbitals are combined to form a hybrid orbital. So, now we will study the hybridization of the molecule $ Xe{F_2} $ . The hybridization of a molecule and the shape of a molecule depends on the central atom of the molecule. So, the central atom in the molecule $ Xe{F_2} $ is xenon $ Xe $ . Now we will write the electronic configuration of Xenon which is $ Xe \to [Kr]4{d^{10}}5{s^2}5{p^6} $ . The electronic configuration of the central atom helps us to find the number of valence electrons. So here according to the electronic configuration of Xenon, we can observe that it has a stable noble gas electronic configuration. So, the number of valence electrons in Xenon is $ 8 $ . Now we will study the chemical structure $ Xe{F_2} $ .

From the above structure we can observe that out of the $ 8 $ electrons, $ 2 $ electrons are bonded with fluorine atoms and the rest six electrons form three electron pairs. Now to predict the hybridization we count the sum of a total number of bonded atoms and the lone pairs over the central atom. So here, the sum is $ 5 $ . So, the hybridization $ Xe{F_2} $ will be $ s{p^3}d $ which means $ 1s $ , $ 3p $ and $ 2d $ orbitals are used. The shape $ Xe{F_2} $ is linear.
Therefore, the correct option is (D).
Note:
The shape of the molecule $ Xe{F_2} $ can be easily explained with the help of its structure. The shape is such that the lone pairs around the central atom acquire the equatorial position and the bond angle is $ {180^0} $ . Therefore, the shape is linear.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




