
The ionic charges on chromate ion and dichromate ion respectively are:
(a)-2, -2
(b)-3, -2
(c)-2, -4
(d)-4, -2
Answer
593.7k+ views
Hint: Ionic charge is the charge on an ion. Ionic charge is due to the gain or loss of one or more electrons from an atom or a group of atoms.
Complete answer:
As mentioned above, ionic charge is the charge on an ion. Let us discuss the two ions mentioned in the question.
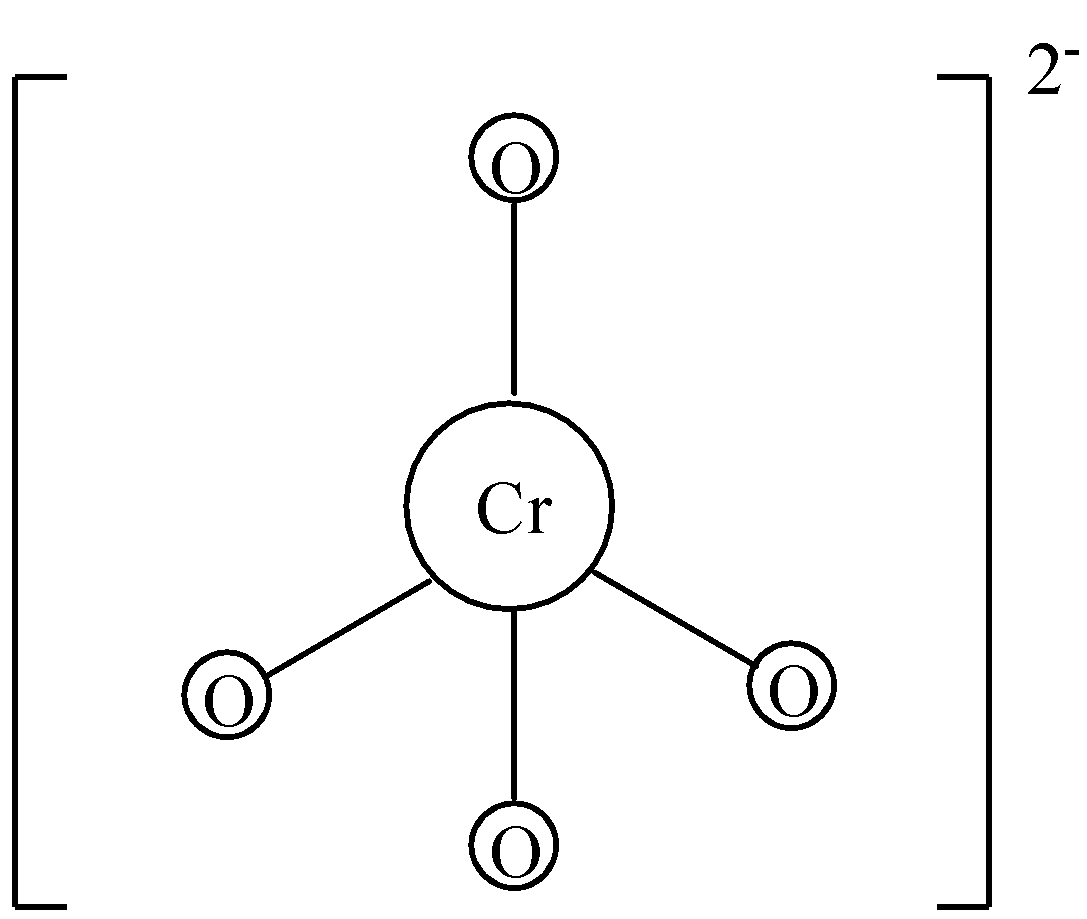
-Chromate ion or chromate anion is present in chromate salts. When chromate salt dissociates, we get chromate ions. It consists of one chromium and four oxygen atoms. It is written as $CrO_{4}^{2-}$. The charge on the chromate ion is -2. The oxidation state of chromium in chromate ion is +6 and the oxidation state of oxygen is -2. There are four oxygen atoms, so we have to multiply -2 with four to get -8. By adding the oxidation state of chromium and total oxidation state of oxygen, we get -2. So, the ionic charge on chromate ions is -2.
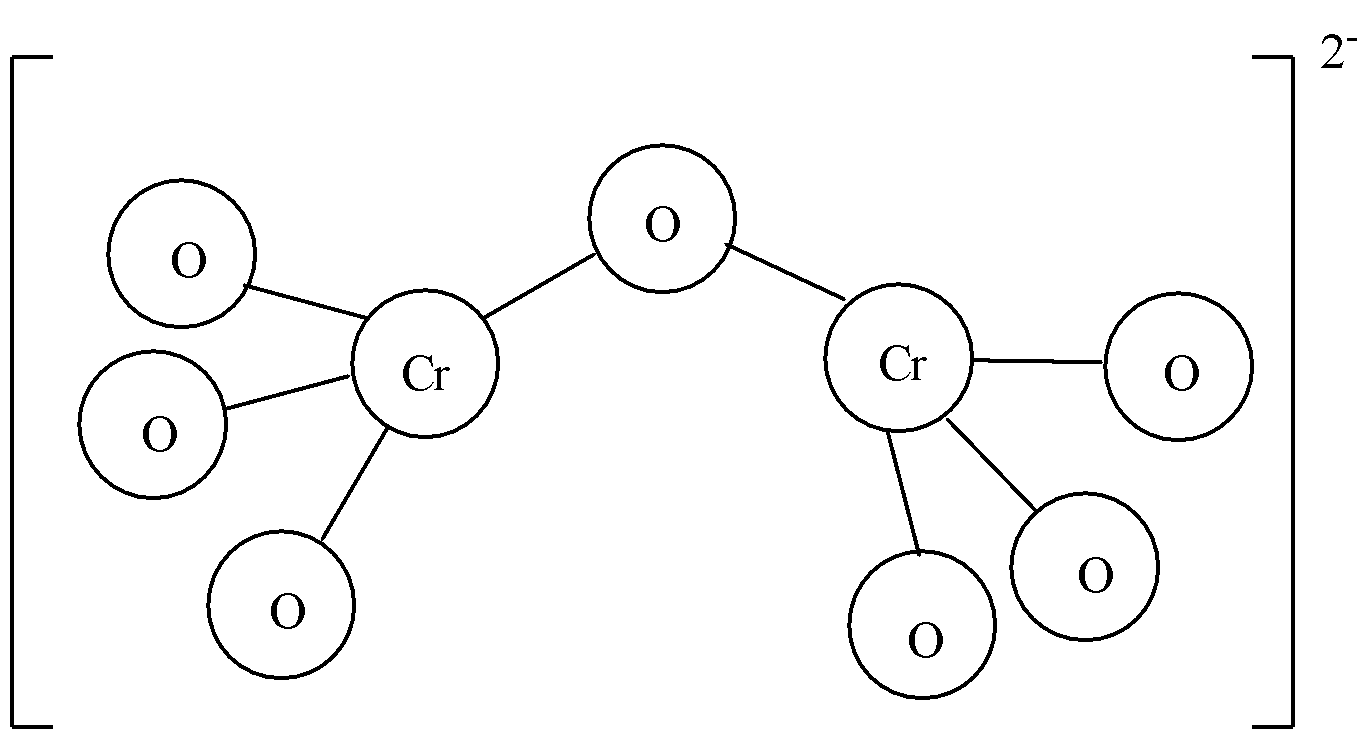
-Dichromate ions are obtained from dissociation of dichromate salts. As the name suggests, there are two chromium atoms in dichromate ion. Dichromate is also an anion. It has an ionic charge of -2. We know that the oxidation state of chromium is +6 in dichromate. There are two chromium atoms, so we need to multiply +6 with two, we get +12. Dichromate anion consists of seven oxygen atoms and oxidation state of oxygen is -2. Multiplying seven with -2 we get -14. Adding the total oxidation state of chromium and oxygen, we get -2. So, the ionic charge on dichromate ions is -2.
Thus, both chromate ion and dichromate ion have ionic charge -2. The correct answer to the question is option (a).
Note: In chromate ion and dichromate ion, the oxidation state of chromium is +6. We already know the oxidation state of oxygen is -2, we can multiply the total number of oxygen present in the molecule with its oxidation state and add it with the oxidation state of chromate ion or dichromate ion. We obtain the total ionic charge of the molecule.
Complete answer:
As mentioned above, ionic charge is the charge on an ion. Let us discuss the two ions mentioned in the question.
-Chromate ion or chromate anion is present in chromate salts. When chromate salt dissociates, we get chromate ions. It consists of one chromium and four oxygen atoms. It is written as $CrO_{4}^{2-}$. The charge on the chromate ion is -2. The oxidation state of chromium in chromate ion is +6 and the oxidation state of oxygen is -2. There are four oxygen atoms, so we have to multiply -2 with four to get -8. By adding the oxidation state of chromium and total oxidation state of oxygen, we get -2. So, the ionic charge on chromate ions is -2.

-Dichromate ions are obtained from dissociation of dichromate salts. As the name suggests, there are two chromium atoms in dichromate ion. Dichromate is also an anion. It has an ionic charge of -2. We know that the oxidation state of chromium is +6 in dichromate. There are two chromium atoms, so we need to multiply +6 with two, we get +12. Dichromate anion consists of seven oxygen atoms and oxidation state of oxygen is -2. Multiplying seven with -2 we get -14. Adding the total oxidation state of chromium and oxygen, we get -2. So, the ionic charge on dichromate ions is -2.

Thus, both chromate ion and dichromate ion have ionic charge -2. The correct answer to the question is option (a).
Note: In chromate ion and dichromate ion, the oxidation state of chromium is +6. We already know the oxidation state of oxygen is -2, we can multiply the total number of oxygen present in the molecule with its oxidation state and add it with the oxidation state of chromate ion or dichromate ion. We obtain the total ionic charge of the molecule.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




