
The IUPAC name of $ C{H_3}C{H_2}COCl $ is:
(A) Propanoyl chloride
(B) Ethanoyl chloride
(C) Acetyl Chloride
(D) Chloroethane
Answer
551.1k+ views
Hint: In chemistry the naming of a compound that is created by two or more different or similar kinds of elements can be a bit complex. However, there are some basic set of rules that are agreed upon, according to which each compound is named. This system of rules is called the IUPAC system. Other than these compounds can also be identified by a common name it holds among people. The longest carbon chain is the parent chain and functional groups are attached as prefixes.
Complete step by step solution:
Naming complex chemical compounds has always been an arguable fact. Out of which naming organic compounds are the most difficult to handle. However, there is a set of rules to make an agreeable chemical name to any complex compound. This nomenclature system is called the IUPAC and is abbreviated as, International union of pure and applied chemistry.
The basic rules of naming organic compounds according to IUPAC are:
It should indicate how many carbon atoms are involved in the organic compound.
It should specify how the carbon atoms are bonded.
It should specify the position and existence of any functional group in the compound.
The root name should specify the major chain of carbon atoms. (The one with a greater number of carbon atoms is treated as the major chain)
The suffix should identify the functional group attached.
The names of other substituent groups are attached as substituents to the name (Make sure to number them such that the substituent chains get the lowest positions).
Now we have the compound, $ C{H_3}C{H_2}COCl $ which can be written as,
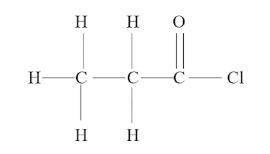
We can see that the longest carbon chain here has 3 carbon atoms, so it should be named “prop “after the number of carbon atoms.
We can also see that the functional group attached is $ = O $ and the substituent here is $ - Cl $ . But we also know that the acid chlorides have a compound formula of $ RCOCl $ . Thus, this is an acid chloride and hence will get the suffix – “only chloride”.
Also, there are only single bonds between the carbon atoms and thus the suffix that gives the nature of carbon-to-carbon bonds will be: “an”
Thus, the compound with formula $ C{H_3}C{H_2}COCl $ will be named according to the IUPAC nomenclature as Propanoyl chloride.
Note:
The number of hydrogen atoms seem to be excluded from the nomenclature, but this is because carbon and hydrogen both are the primary composition in an organic compound. However, hydrogen atoms usually define and satisfy the tetravalency of the carbon atoms in the structure.
Complete step by step solution:
Naming complex chemical compounds has always been an arguable fact. Out of which naming organic compounds are the most difficult to handle. However, there is a set of rules to make an agreeable chemical name to any complex compound. This nomenclature system is called the IUPAC and is abbreviated as, International union of pure and applied chemistry.
The basic rules of naming organic compounds according to IUPAC are:
It should indicate how many carbon atoms are involved in the organic compound.
It should specify how the carbon atoms are bonded.
It should specify the position and existence of any functional group in the compound.
The root name should specify the major chain of carbon atoms. (The one with a greater number of carbon atoms is treated as the major chain)
The suffix should identify the functional group attached.
The names of other substituent groups are attached as substituents to the name (Make sure to number them such that the substituent chains get the lowest positions).
Now we have the compound, $ C{H_3}C{H_2}COCl $ which can be written as,

We can see that the longest carbon chain here has 3 carbon atoms, so it should be named “prop “after the number of carbon atoms.
We can also see that the functional group attached is $ = O $ and the substituent here is $ - Cl $ . But we also know that the acid chlorides have a compound formula of $ RCOCl $ . Thus, this is an acid chloride and hence will get the suffix – “only chloride”.
Also, there are only single bonds between the carbon atoms and thus the suffix that gives the nature of carbon-to-carbon bonds will be: “an”
Thus, the compound with formula $ C{H_3}C{H_2}COCl $ will be named according to the IUPAC nomenclature as Propanoyl chloride.
Note:
The number of hydrogen atoms seem to be excluded from the nomenclature, but this is because carbon and hydrogen both are the primary composition in an organic compound. However, hydrogen atoms usually define and satisfy the tetravalency of the carbon atoms in the structure.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




