
The IUPAC name of the compound is:
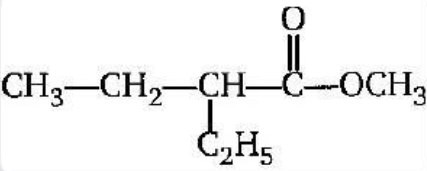
(A) Methyl-2-ethyl butanoate
(B) 1-methoxy-2-ethyl butanoate
(C) 3-methoxycarbonyl pentane
(D) 1-methoxy-2-ethyl butanal

Answer
583.8k+ views
HintTo write the IUPAC nomenclature we have to understand the compound structure and the functional group attached to it. For the IUPAC naming of compounds, there are certain rules to be followed. The IUPAC nomenclature is a stepwise rule to name the compound.
Complete step by step solution:
IUPAC stands for the international union for pure and applied chemistry at the steps to write the IUPAC nomenclature are mentioned below:
Step 1- Select the longest carbon chain – the longest carbon chain in the given compound is the carbon chain of 5 carbons hence pent will be used to denote the carbon chain.
Step-2 Identify the substituents attached to the parent chain and number the parent chain from the end which gives the lowest number to the substituent- in the given compounds the substituents attached is ethyl and the lowest number of ethyl substituent is 2, the given compound is a methyl ester of butanoic acid.
Hence the IUPAC name of the given compound is:
Methyl-2-ethyl butanoate
Hence the correct answer is option (A).
Note: While writing the IUPAC nomenclature the substituents present in the given compound should be arranged in alphabetical order and if the same substituent occurs more than one then the prefix like di, tri, tetra etc. are used. It is important to put a comma between two numbers and a dash between a number and the name of the substituent. While arranging the substituents in alphabetical order we should ignore the prefix like di, tri, and tetra and arrange on the basis of the first letter of the substituent name.
Complete step by step solution:
IUPAC stands for the international union for pure and applied chemistry at the steps to write the IUPAC nomenclature are mentioned below:
Step 1- Select the longest carbon chain – the longest carbon chain in the given compound is the carbon chain of 5 carbons hence pent will be used to denote the carbon chain.
Step-2 Identify the substituents attached to the parent chain and number the parent chain from the end which gives the lowest number to the substituent- in the given compounds the substituents attached is ethyl and the lowest number of ethyl substituent is 2, the given compound is a methyl ester of butanoic acid.
Hence the IUPAC name of the given compound is:
Methyl-2-ethyl butanoate
Hence the correct answer is option (A).
Note: While writing the IUPAC nomenclature the substituents present in the given compound should be arranged in alphabetical order and if the same substituent occurs more than one then the prefix like di, tri, tetra etc. are used. It is important to put a comma between two numbers and a dash between a number and the name of the substituent. While arranging the substituents in alphabetical order we should ignore the prefix like di, tri, and tetra and arrange on the basis of the first letter of the substituent name.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




