
The IUPAC name of the crotonaldehyde is
A.2-Propenal
B.Propenal
C.2-Butenal
D.Butenals
Answer
582.6k+ views
Hint: The molecular formula of crotonaldehyde is ${{\text{C}}_{\text{4}}}{{\text{H}}_{\text{6}}}{\text{O}}$ . It is an unsaturated aldehyde. Crotonaldehyde is the common name of the compound. Use the IUPAC rules to determine the IUPAC name of the compound.
Step by step answer: The molecular formula of crotonaldehyde is ${{\text{C}}_{\text{4}}}{{\text{H}}_{\text{6}}}{\text{O}}$ and its structure is as follows:
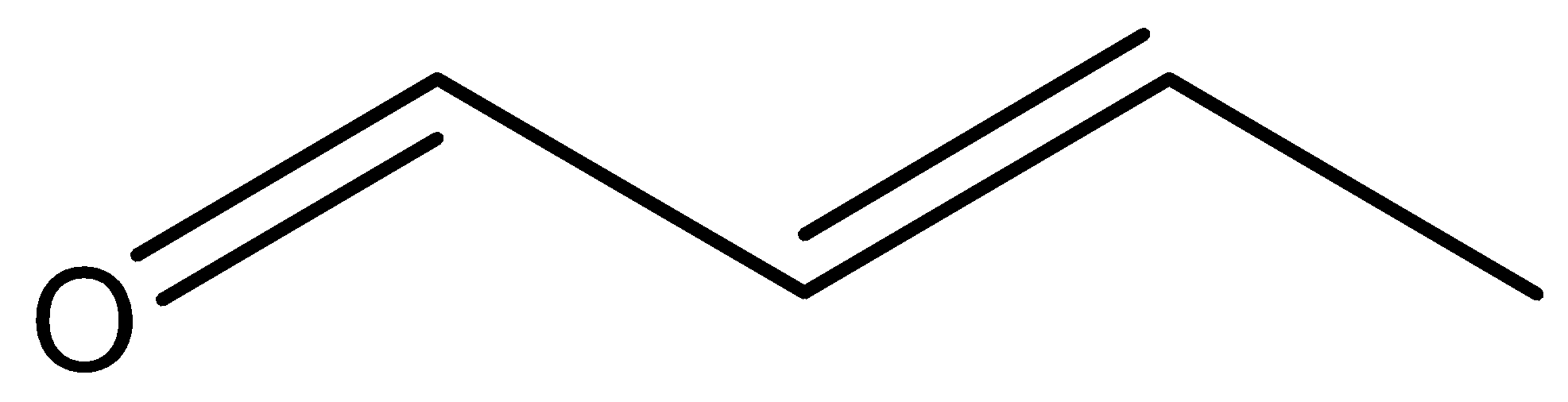
Using the IUPAC rule determines the IUPAC name of the crotonaldehyde.
Rule1. Determine the longest continuous chain (parent chain) of carbon atoms.
In the structure of crotonaldehyde, the longest continuous chain contains 4 carbon atoms.
Rule 2. Number the chain in such a way that the functional group will get the lowest priority.
In crotonaldehyde, aldehyde is the functional so number the parent chain from the carbon atom where the carbonyl group ${\text{(C = O)}}$ is bonded.
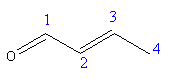
As the parent chain contains 4 carbon atoms with aldehyde functional group so the root name of the compound is butanal. However, the compound also contains ${\text{C = C}}$ at position 2 so the IUPAC name of the crotonaldehyde is 2-butenal.
As the longest continuous chain contains 4 atoms so option (A) 2-Propenal is incorrect as it indicates the parent chain contains 3 carbon atoms.
Similarly, option (B) Propenal is also incorrect as it indicates; the parent chain contains 3 carbon atoms.
Option (D) Butenals is incorrect as the position of the double bond is not mentioned in the name of the compound.
Thus, the correct option is (C) 2-butenal.
Note: The IUPAC naming is the systematic approach of naming the compounds. Few IUPAC rules are common for all compounds while few rules get changed as per the functional groups. In general, the IUPAC system of naming an organic compound consists of prefix and suffix. The prefix depends on the number of carbon atoms present in a parent chain and the suffix denotes the functional group present in the compound. If there is more than one functional group like aldehyde and alkene in this case use the suffix of a functional group having the highest priority.
Step by step answer: The molecular formula of crotonaldehyde is ${{\text{C}}_{\text{4}}}{{\text{H}}_{\text{6}}}{\text{O}}$ and its structure is as follows:

Using the IUPAC rule determines the IUPAC name of the crotonaldehyde.
Rule1. Determine the longest continuous chain (parent chain) of carbon atoms.
In the structure of crotonaldehyde, the longest continuous chain contains 4 carbon atoms.
Rule 2. Number the chain in such a way that the functional group will get the lowest priority.
In crotonaldehyde, aldehyde is the functional so number the parent chain from the carbon atom where the carbonyl group ${\text{(C = O)}}$ is bonded.

As the parent chain contains 4 carbon atoms with aldehyde functional group so the root name of the compound is butanal. However, the compound also contains ${\text{C = C}}$ at position 2 so the IUPAC name of the crotonaldehyde is 2-butenal.
As the longest continuous chain contains 4 atoms so option (A) 2-Propenal is incorrect as it indicates the parent chain contains 3 carbon atoms.
Similarly, option (B) Propenal is also incorrect as it indicates; the parent chain contains 3 carbon atoms.
Option (D) Butenals is incorrect as the position of the double bond is not mentioned in the name of the compound.
Thus, the correct option is (C) 2-butenal.
Note: The IUPAC naming is the systematic approach of naming the compounds. Few IUPAC rules are common for all compounds while few rules get changed as per the functional groups. In general, the IUPAC system of naming an organic compound consists of prefix and suffix. The prefix depends on the number of carbon atoms present in a parent chain and the suffix denotes the functional group present in the compound. If there is more than one functional group like aldehyde and alkene in this case use the suffix of a functional group having the highest priority.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

A moving boat is observed from the top of a 150 m high class 10 maths CBSE




