
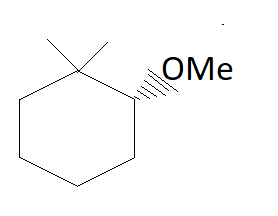
The major product of the reaction is:

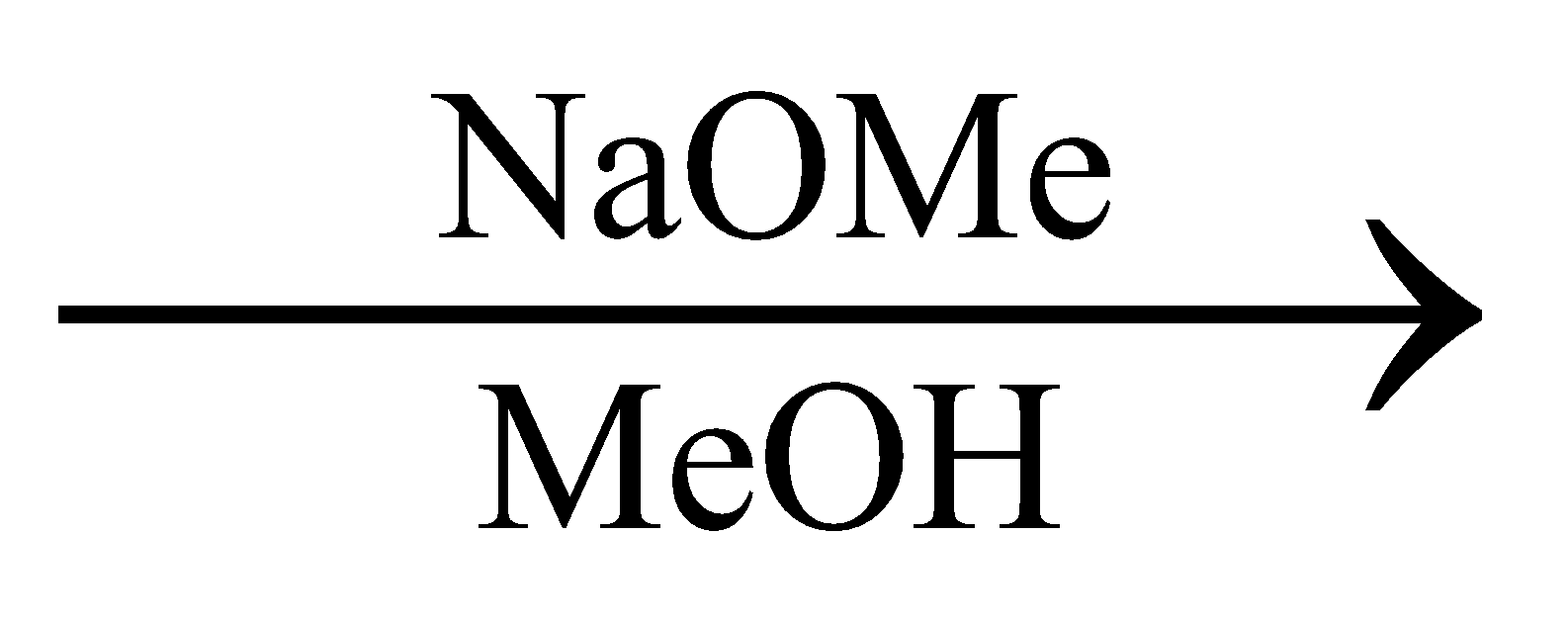
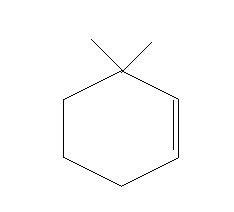
A.
B.
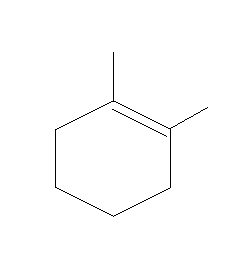
C.
D.

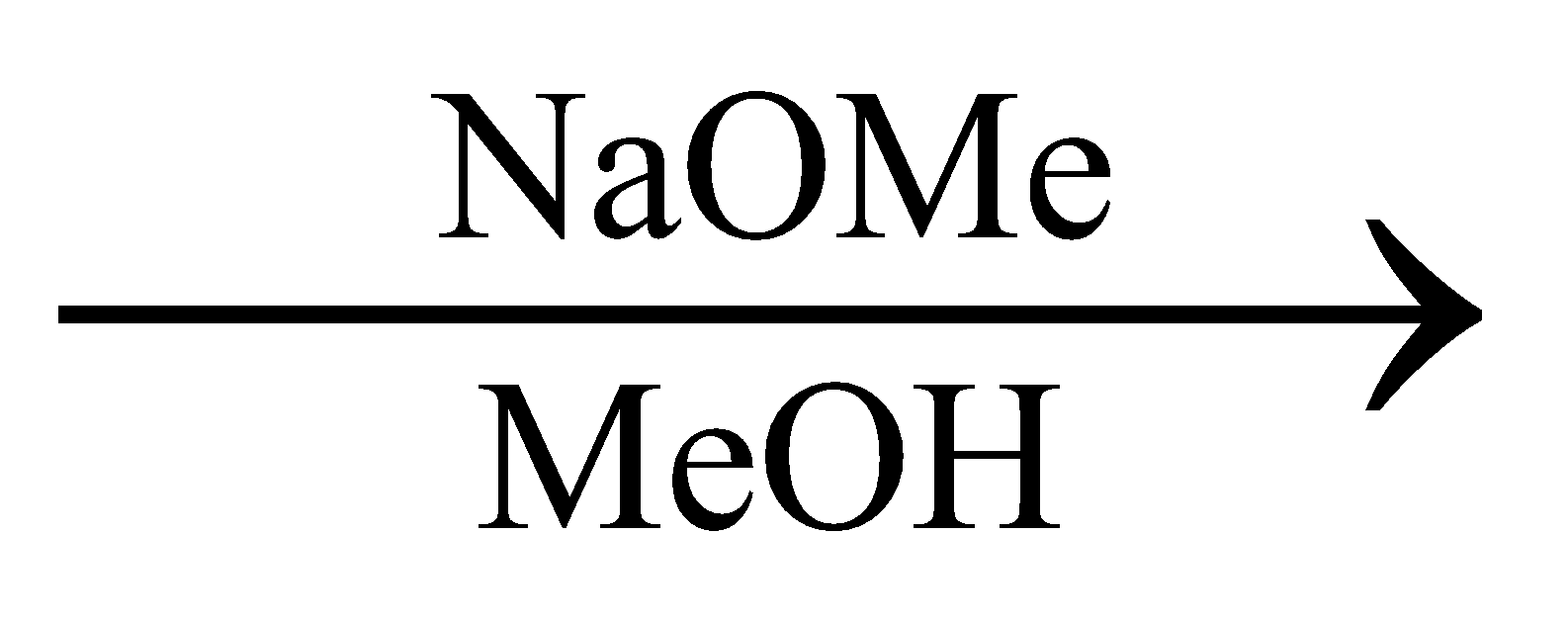




Answer
583.8k+ views
Hint: The above reaction given in the question is a type of elimination reaction. An elimination reaction is a type of reaction in which two substituents are removed from a molecule in either one-step or two-step mechanism. The one-step mechanism is known as the E2 mechanism and two-step mechanism is known as the E1 mechanism.
Complete step by step answer:
In the above reaction in the presence of NaOMe elimination of HBr occurs.
Let us assume that the carbon to which Br is attached is C1, the Carbon just below is C2, As shown in the following diagram:
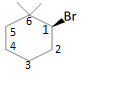
In the presence of NaOMe, Br will be eliminated from C1 generating a positive charge on C1.
Now, since during an elimination reaction two substituents from adjacent carbon are removed, in addition to Br, there must be an elimination of another substituent as well.
It can be the elimination of H from C2 or elimination of methyl groups from C5. Since the removal of H is easier as compared to removal of the Methyl group, H from C2 will be eliminated. And a negative charge will be generated at C2
This will lead to the formation of a double bond between C1 and C2.
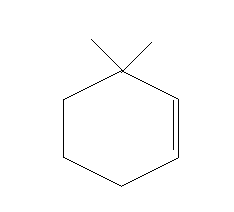
This implies, that the major product of the given reaction will
Thus, option A is the right answer.
Note:
There are three types of organic reactions, namely
-Addition reaction: a molecule gets added to the other molecule. For example, the addition of hydrogen to ethane.
-Elimination reaction: discussed above
-Substitution reaction: a substituent of a molecule gets replaced by another substituent. For example, when chloromethane reacts with NaOH, methanol is formed and Cl gets replaced by the OH group.
Complete step by step answer:
In the above reaction in the presence of NaOMe elimination of HBr occurs.
Let us assume that the carbon to which Br is attached is C1, the Carbon just below is C2, As shown in the following diagram:

In the presence of NaOMe, Br will be eliminated from C1 generating a positive charge on C1.
Now, since during an elimination reaction two substituents from adjacent carbon are removed, in addition to Br, there must be an elimination of another substituent as well.
It can be the elimination of H from C2 or elimination of methyl groups from C5. Since the removal of H is easier as compared to removal of the Methyl group, H from C2 will be eliminated. And a negative charge will be generated at C2
This will lead to the formation of a double bond between C1 and C2.

This implies, that the major product of the given reaction will
Thus, option A is the right answer.
Note:
There are three types of organic reactions, namely
-Addition reaction: a molecule gets added to the other molecule. For example, the addition of hydrogen to ethane.
-Elimination reaction: discussed above
-Substitution reaction: a substituent of a molecule gets replaced by another substituent. For example, when chloromethane reacts with NaOH, methanol is formed and Cl gets replaced by the OH group.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




