
What will be the molecular formula of a polypeptide consisting of 10 glycine molecules when the formula of glycine is ${{C}_{2}}{{H}_{5}}{{O}_{2}}N$?
(A) ${{C}_{6}}{{H}_{12}}O{{N}_{5}}$
(B) ${{C}_{20}}{{H}_{32}}{{O}_{11}}{{N}_{10}}$
(C) ${{C}_{30}}{{H}_{16}}{{O}_{6}}{{N}_{10}}$
(D) ${{C}_{25}}{{H}_{16}}{{O}_{6}}{{N}_{5}}$
Answer
573.6k+ views
Hint: An attempt to this question can be made by understanding the meaning of peptides and polypeptides. Now draw the expanded structure of glycine. Upon your understanding of peptides, perform the chemical reaction on 10 molecules of glycine and then write the compressed form of the product. With this you can arrive at the correct answer.
Complete Solution :
- Peptide is defined as a short chain of amino acids.
- The amino acids in a peptide are usually connected to each other in a sequence of bonds also called the peptide bonds. Peptides of relatively longer length of chain are termed as polypeptides.
- In the process, the carboxylic group attaches to the amine group of the next molecule of amino acid releasing one molecule of water. Thus, peptide formation is a type of condensation reaction.
- We will now draw the expanded structure of the amino acid, glycine.
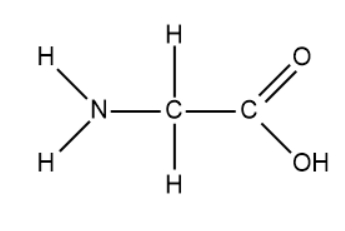
- We will now perform the condensation reaction on 10 molecules of glycine. It is important to know that the peptide bond is an amide.
The reaction is given below:
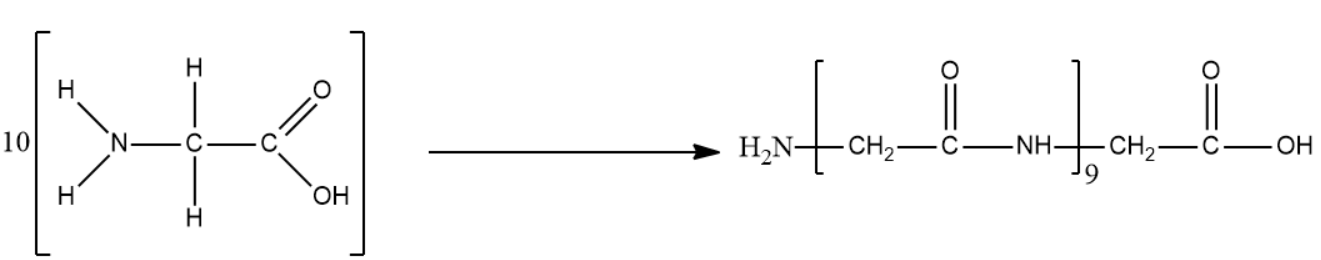
The condensed form of the above-mentioned product is ${{C}_{20}}{{H}_{32}}{{O}_{11}}{{N}_{10}}$.
So, the correct answer is “Option B”.
Note: Amino acids get their name due to the functional group present in a molecule of amino acids. They have an amine group as well as a carboxylic acid group which is a unique feature. This is because one molecule has an acidic group as well as an alkaline group.
Complete Solution :
- Peptide is defined as a short chain of amino acids.
- The amino acids in a peptide are usually connected to each other in a sequence of bonds also called the peptide bonds. Peptides of relatively longer length of chain are termed as polypeptides.
- In the process, the carboxylic group attaches to the amine group of the next molecule of amino acid releasing one molecule of water. Thus, peptide formation is a type of condensation reaction.
- We will now draw the expanded structure of the amino acid, glycine.

- We will now perform the condensation reaction on 10 molecules of glycine. It is important to know that the peptide bond is an amide.
The reaction is given below:

The condensed form of the above-mentioned product is ${{C}_{20}}{{H}_{32}}{{O}_{11}}{{N}_{10}}$.
So, the correct answer is “Option B”.
Note: Amino acids get their name due to the functional group present in a molecule of amino acids. They have an amine group as well as a carboxylic acid group which is a unique feature. This is because one molecule has an acidic group as well as an alkaline group.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




